Chromatography
Different types of chromatography techniques
The chromatography technique is one of the most powerful methods for separating a sample, such as a synthesized mixture or a biological crude extract, into its single components. The chromatography separation technique is based on substances partitioning between two phases: a stationary phase with a large surface and a mobile phase which moves through the stationary phase.
The most frequently used types of chromatography are gas or liquid chromatography. The difference is related to the physical state of the mobile phase in column chromatography. In gas chromatography, the mobile phase is a gas which transports the sample through a solid stationary phase, whereas in liquid chromatography the mobile phase is a solvent. The interaction of the compounds with the stationary phase, a process known as the mode of separation, is governed by differences in polarity, size, or specific binding affinities. The mode of chromatography method of separation determines the type of liquid chromatography technique (Table 1).
Table 1. Types of liquid chromatography
| Types of liquid chromatography | Mode of chromatography separation based on: |
| Adsorption chromatography (Normal- and reversed-phase chromatography) | Polarity |
| Affinity chromatography | Specific binding interaction |
| Size exclusion chromatography | Molecular sizes |
| Ion exchange chromatography | Charge |
Chromatography purification workflow
Adsorption column chromatography is the main part of a typical isolation and purification workflow for drugs, chemicals, flavors and others. Initially, the substances are chemically synthesized or extracted from plants, bacteria or other living organisms. Evaporation is used to concentrate the material to simplify downstream processing. If the sample is new and unfamiliar, a screen for optimal chromatography separation conditions is performed, usually by thin-layer chromatography (TLC) or analytical high-pressure liquid chromatography (HPLC methods. Once suitable conditions have been found, the chromatographic process is upscaled to preparative chromatography. In the preparative step, the target compound is purified in high quantities either by flash chromatography, prep HPLC or a combination of both. If the techniques are used together, flash chromatography is applied for the pre-purification step and prep HPLC to achieve final high purity. After successful separation of compounds, a second concentration step is performed either by evaporation or freeze drying. At this point, the compound is ready for analysis of its purity and function by other techniques, including melting point analysis, analytical HPLC, enzymatic assays and others.
The complete workflow after synthesis or extraction is shown in Fig. 1.

Figure 1. The purification workflow in a general extraction or synthesis workflow
Adsorption column chromatography was the very first form of liquid chromatography, discovered more than 100 years ago by Mikhail Tsvet, a Russian-Italian botanist who used this type of chromatographic process to separate the pigments in plants. Since then, the chromatography technique has developed very rapidly into an essential method used in synthesis and extraction labs.
In adsorption chromatography, the separation is governed by the interactions of the sample components with the stationary and mobile phase. Compounds with different chemical properties (polarities) display varying affinities, or strengths of adhesion, towards the mobile phase and stationary phase. Affinity is influenced by two molecular properties, adsorption and desorption. Adsorption refers to the ability of a certain component to stick to the stationary phase. Desorption, or solubility, describes how well a component of the mixture dissolves in the mobile phase. The speed with which individual sample components migrate through the stationary phase depends on their adsorption/desorption properties as shown in Fig. 2.
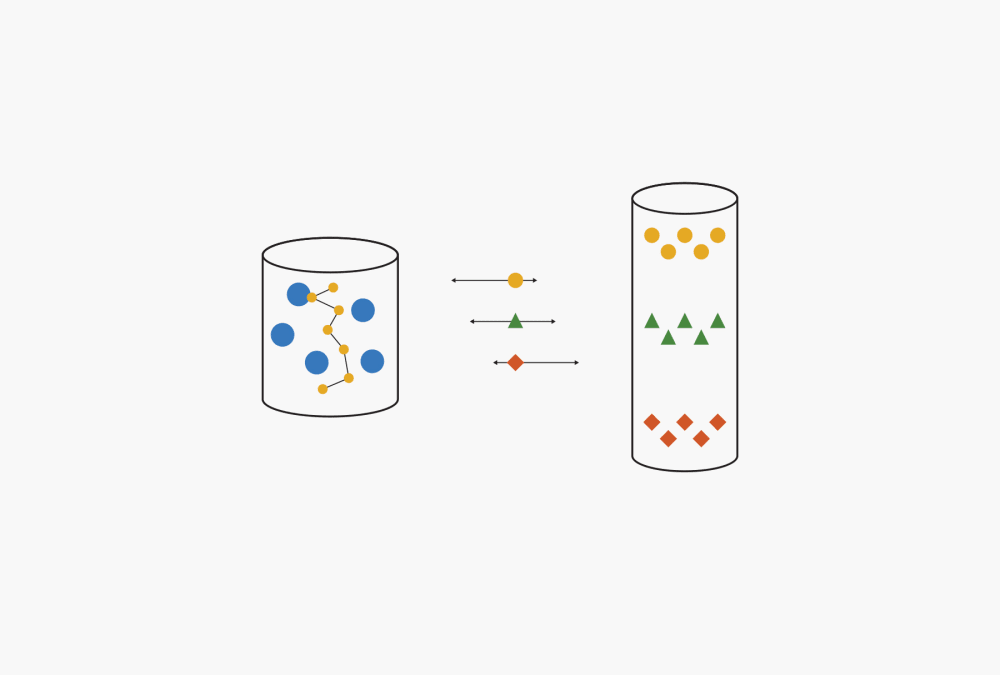
Figure 2: Adsorption versus desorption
Flash chromatography vs the prep HPLC technique
The first publication using elevated pressure came out at the end of the seventies with a method called flash chromatography. In addition to this, attempts were made to increase the size of the analytical HPLC systems and thus make them available also for preparative chromatography (prep HPLC ). Nowadays, both techniques are frequently used but for different objectives: flash chromatography is mainly used as a pre-purification step to purify large sample quantities at a decent resolution, whereas in prep HPLC the goal is to achieve highest resolution (purity) under the condition of lower loading capacities.
Therefore, the two chromatography techniques differ in the material used for the stationary phase (different particle size), the dimension of the cartridge or column (internal diameter (ID) and lengths) and flow rate of the mobile phase as shown in Table 2.
Table 2. Differences between flash chromatography and prep HPLC
Flash chromatography | Prep HPLC | |
|---|---|---|
Particle size | 15 – 63 µm | 5 – 15 µm |
Column ID | 12 – 115 mm | 10 – 70 mm |
Flow rate | 15 – 250 mL/min | 5 – 100 mL/min |
Loading Capacity | < 300 g | < 10 g |
Maximum pressure | 50 bar | 300 bar |

