Rotary Evaporation
Rotary Evaporation technique & Distillation process
The distillation process is used to remove volatile solvents from liquid mixtures through vaporization and subsequent condensation. In the lab, chemists and biochemists frequently use the distillation process and the rotary evaporator.
Distillation history
The historical development of rotary evaporation, or «drop-by-drop separation», began thousands of years ago. A rich history of how the distillation process has evolved over time is presented in the table below.
| 3,500 BC | Persians invent distillation to produce rose water. The distillation technique spread quickly across Europe, North Africa and Asia. The distillation process was used to produce essences, desalinate seawater and for alchemy. |
| 2nd century | As popularity of alchemy, a combination of religious aspects and chemistry, grew, the search for “the prima materia”, a fundamental material with no qualities, intensified. Alchemists sought to transform natural materials chemically into this basic material and then to impart the basic material with new desired qualities, such as those of gold. In their trials, they discovered many chemical compounds, improved existing processes and equipment and discovered new methods that are still used in modern chemistry today. They also developed distillation technology that, from a design-related standpoint, is still used today. Four standard components of this distillation equipment include the heating bath, the bubble flask, the head, and the condenser. |
| 17th and 18th centuries | Focus was placed on improving existing distillation technology. The distillation equipment was insulated, the apparatus was increasingly made out of glass, rather than metal, the process of continuous distillation was introduced, and water was used as a coolant. The vapor distillation process was also discovered during this time. At the end of the 17th century, the Irish physicist Robert Boyle (1627-1691) performed the first vacuum distillations. |
| 19th century | The first rectifying columns were invented to make multi-stage distillation possible. With the arrival of organic chemistry, new distillation equipment was specifically designed for the needs of the laboratory. Financial involvement by the alcohol industry in France brought about a rapid development on a large industrial scale as well. The invention of the pressure regulator and improvement of pumps also enabled a more directed use of the vacuum. |
| 1950 - 1955 | Articles by C.C. Draig (1950) and M.E. Volk (1955) published the operating principle behind the rotary evaporator. This process has a far better heat transfer rate than the flask process, which spares the product and increases output. |
| 1957 | BÜCHI Labortechnik in Flawil brought the first rotary evaporator to the market. |
The distillation process and related rotary evaporation technology
The rotary evaporator has been created to respond to the need of chemists and biochemists around the world. Due to the broad range of condensers, the Rotavapor® is used for fast distillation of mixed solvents, efficient drying of samples, quicker freeze-drying sample preparation, chemical synthesis under reflux, extraction of natural compounds and concentration. The industrial applications of the rotary evaporator are countless, but include crude oil processing, separation of cannabinoids, molecular cooking, flavor and fragrance creation and many more.
Evaporation in the distillation process
Evaporation is the shift of a particle from the liquid phase to the gaseous phase. The evaporation process starts as soon as the conditions for pressure and temperature reach the boiling curve. At this point, all the particles have enough kinetic energy as an energy requirement to overcome the forces of mutual attraction binding them. It is now no longer a question of just a few molecules on the surface, breaking away from the liquid. The shift from liquid to gas is now taking place throughout the liquid. The boiling point is essential for distilling because the liquid to be removed vaporizes far more quickly than during evaporation. Because the molar volume of a gas is several times greater than that of a liquid, the material expands 1,000- to 2,000-fold during boiling. Care must be taken to ensure that the distillation equipment can handle this volume.
Nucleate boiling during the evaporation process
The first stage in boiling during the distillation process is nucleate boiling. The heat causes bubbles of gas enclosed within the walls of the container to start expanding. The vaporized particles leave the liquid and go into these bubbles, causing them to grow. Once the bubbles reach a point where their buoyancy can overcome the forces of adhesion, they break away incompletely from the wall of the vessel and rise to the surface. The remaining part of the bubble serves as the germ for a next bubble at the same point. Adrift flow forms behind the bubble, improving mixing within the liquid. As the liquid becomes warmer, more and more bubbles form until, finally, there is an uninterrupted film of vapor covering the wall of the vessel. This stage refers to film boiling.
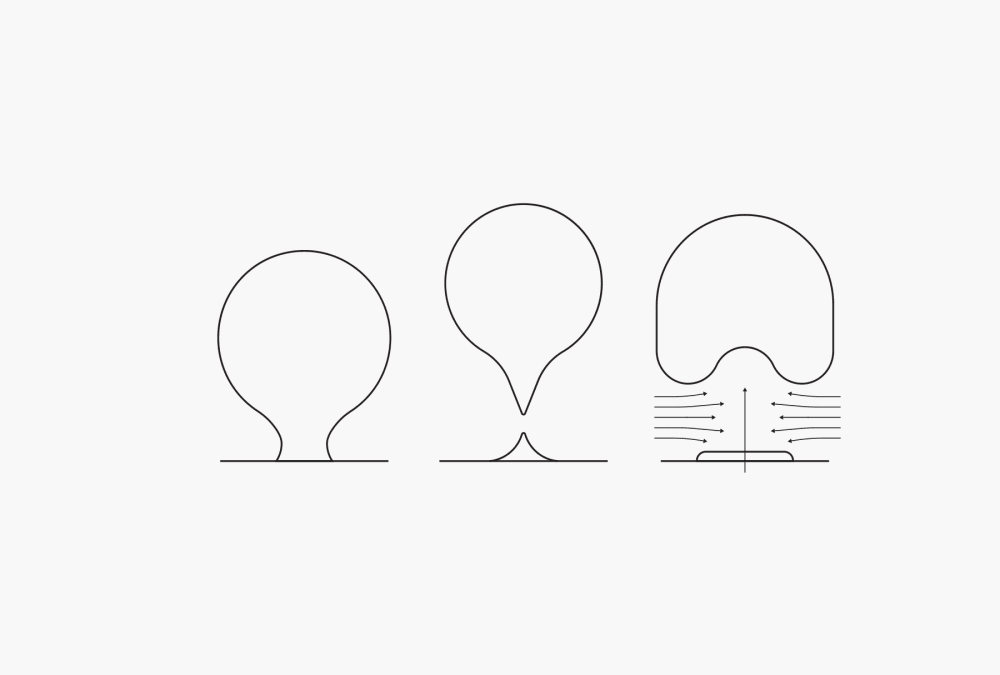
Figure 1. Nucleate boiling
Transfer of heat during the evaporation process
One important aspect of boiling is the transfer of heat from the heat source to the liquid. Because the liquid generally comes into contact with the heat source only at the wall of the container, its outer layers become warm first. The top layers remain colder. The warm layers rise due to convection, and the colder layers take their place. This brings about an equalization of temperatures but progresses very slowly. The additional mixing at the start of nucleate boiling does improve the heat transfer, but the situation is still unsatisfactory. The heat transfer can be enhanced considerably by keeping the liquid in motion with a mixer or in a rotating flask using rotary evaporation technology. This continual mixing or forced convection enables excellent heat transfer, better expulsion of the gas form, and thus a quicker distillation process.
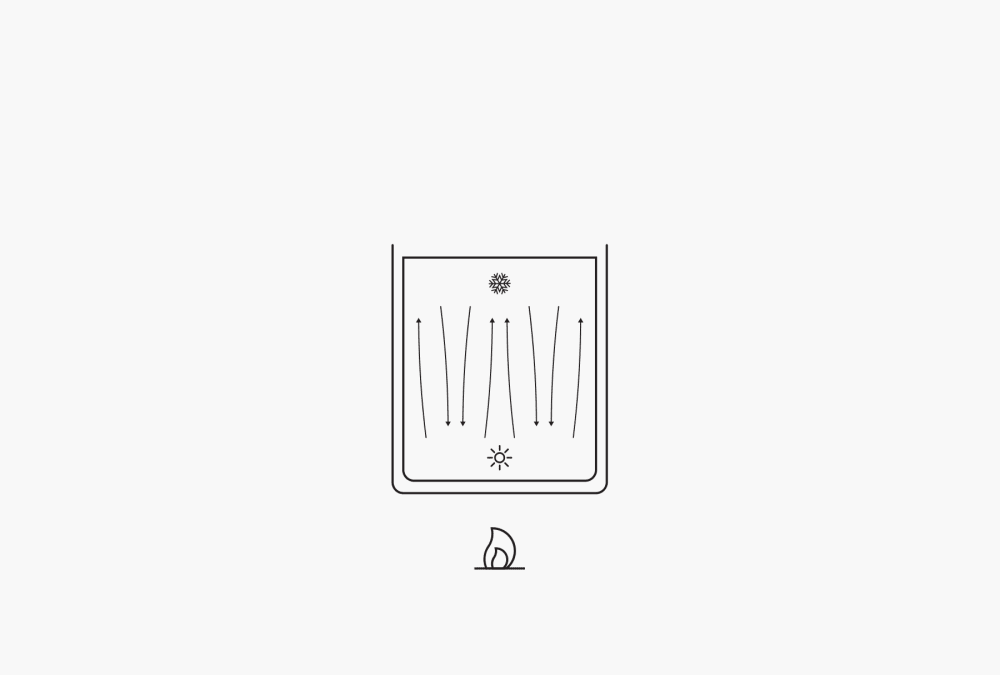
Figure 2. Free convection
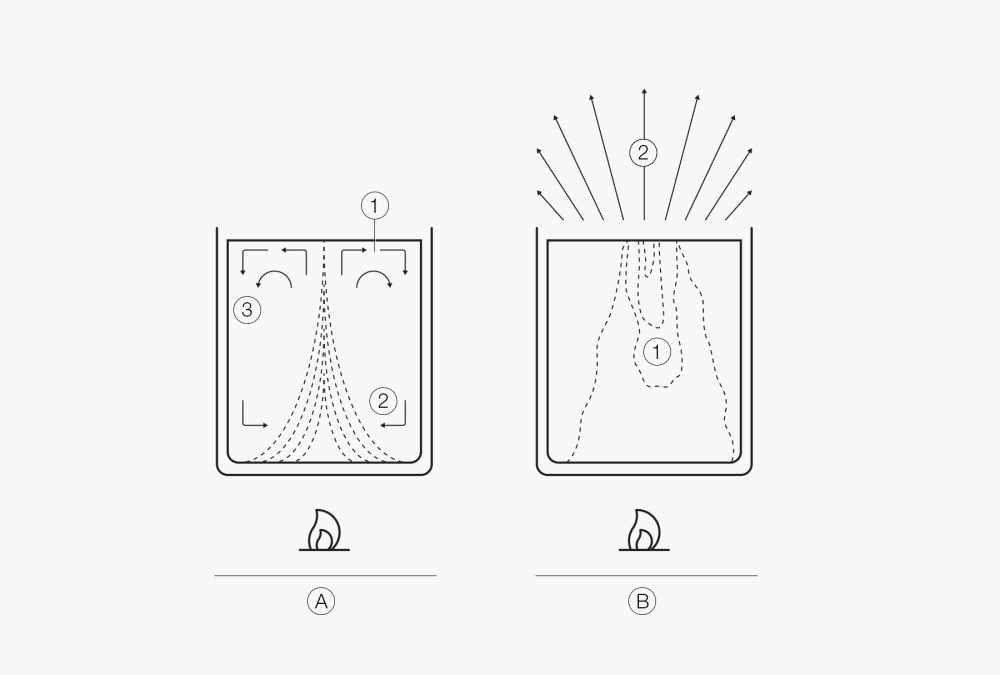
Figure 3:
Start of retarded boiling (1. Bumping), (2. Temp. Profile, 3. Convection)
Retarded boiling (1. Bumping) at the surface.
Condensation in the distillation process
Condensation is the reversal of this boiling process in which a substance transforms from its gaseous into its liquid state. Since the heat of evaporation transferred to the particles during the boiling must now be removed from the particles, cooling is required to condense a gas.
The vapor leaving the point of vaporization reaches the condensation section. Because the temperature of the condenser is lower than the condensation temperature of the vapor, the vapor precipitates out and a film of liquid forms immediately as its molecules strike the condenser. Since this film impedes the heat transfer, provisions must be taken to ensure that it can flow off. Condensers therefore always have a vertical or diagonal design. The condensate flowing off collects in a collection flask. Because the volume of the gas being condensed is considerably greater than that of the liquid produced, the heat cannot be carried off easily. This is why coolers usually have very large surface areas.
To ensure you can condense effectively throughout the entire distillation process, the work is done using a cooling medium that can be continually refreshed, e.g., flowing tap water, or a recirculating chiller. Boiling results in a great increase in pressure. In condensation, a very great deal of pressure is dissipated. The condenser acts as a pump.
How gas is transported through the rotary evaporator
A distillation consists of a vaporization and a subsequent condensation. Because the points where the vaporization and the condensation occur are usually well separated, the vapor must be transported. This can be easily done. Because a gas distributes itself evenly within the space available, it flows from the evaporator side to the condenser side, where it liquefies. This produces an equivalent drop in volume. A local vacuum results. The condenser side therefore always draws gas, while the evaporator side always supplies the same amount of gas. The dynamic difference in pressure created moves the vapor through the apparatus at high speeds. The force maintaining this flow is the heat of evaporation supplied to the gas during vaporization. During condensation this heat is withdrawn again from it. This phenomenon is therefore also referred to as a thermal pump (P).
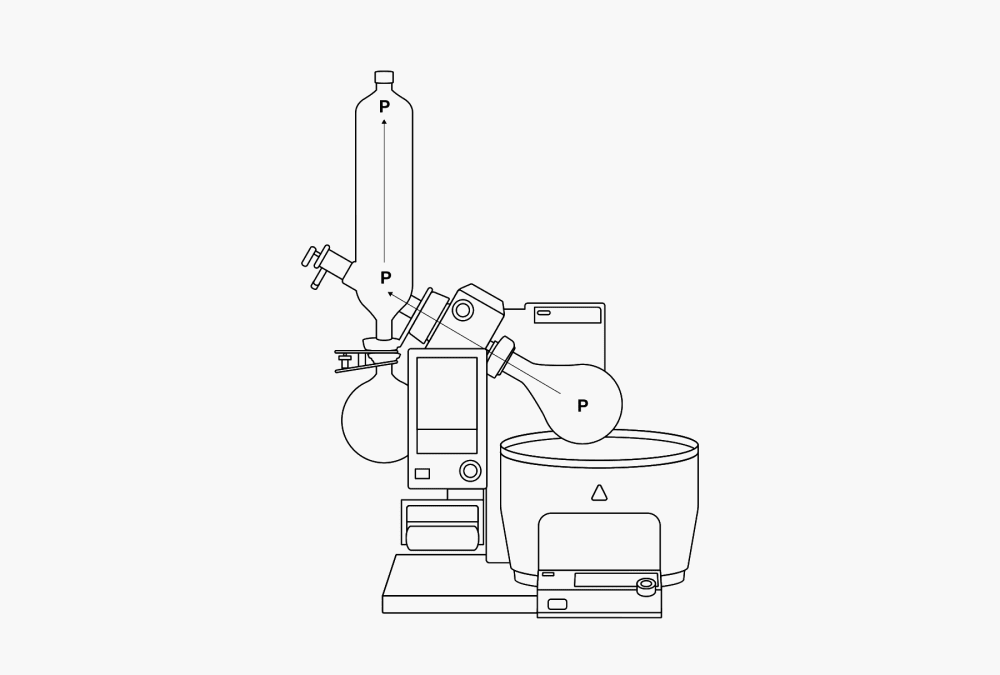
Figure 4. The thermal pump (P) and local differences in temperature within the rotary evaporator.
In order to maintain a balanced difference in dynamic pressure, it is important that the speed of condensation and the speed of evaporation be attuned to one another. Whenever more substance is being vaporized than is being condensed in the condenser, the pressure within the apparatus will rise and the vacuum pump will have to draw continually, pulling in the vaporized solvent and pumping it out into the environment. It is best to work with a boiling temperature about 20 °C higher than the temperature of the cooling water. This ensures that the heat balance will be kept in equilibrium.
Separation by distillation process
Distillation is a separation technique for separating mixtures made up of two liquids. The distillation process is based on the difference between the vapor pressures of the substances. The mixture is heated until it is vaporized and is then re-condensed. In this process, the more volatile component builds up in the vapor, and thus in the condensate as well, resulting in separation. The enriched vapor, directed through the distillation equipment, reaches the condenser where it liquefies and is gathered in a collection vessel as a distillate. At the same time, the less volatile component accumulates in the evaporating flask.
If the boiling points of two materials differ by more than 80 °C, separating mixtures can be achieved with a single distillation. Single distillation is used mainly to separate highly volatile solvents from high boiling materials. It makes no difference here whether it is the solvent (cleaning of solvents) or the residue (cleaning of a product of reaction by solvent removal) that is to be regained. Whenever the boiling points of the two components being separated are too close together, the distillation process must be repeated several times. This procedure is called rectification. Alternatively, fractional distillation can be used to separate two liquids with a small difference in boiling temperatures. In fractional distillation, a fractionating column, filled with glass or plastic beads, is placed between the boiling flask and the condenser. The glass beads in the fractionating column provide more surface on which the liquid can condense, re-evaporate and condense again.
The role of the vacuum in the distillation process
Vacuum plays an important role in connection with all types of evaporators because it lowers the boiling temperature needed for the distillation. The vacuum may be controlled either manually or automatically if a Vacuum Controller has been installed. The vacuum is built up in a vacuum source outside the rotary evaporator. This is either a pump in the laboratory, often a water jet pump or a diaphragm pump, or an in-house vacuum line. The operation of a laboratory pump can be regulated with a Vacuum Controller, which saves water, electricity and increases the lifespan of the pump.
The Rotavapor® is evacuated and re-aerated across the vacuum connection on the glass assembly. The location of this connection on the equipment is of importance. It must be at the area where the local overpressure built up by the vaporization is being dissipated again by the condensation. This is at the very top of a rising cooler and at the very bottom of a descending cooler.
