The detectives shine a light on Kjeldahl – Part 2: Steam distillation & Titration

Chapter 41
? Workshop overview: The detectives continue their in-depth investigation into the age-old laboratory process – the Kjeldahl method. Having heard Shallot Holmes and Eggcule Poirot explain the importance of sample preparation and the digestion stage. It is now up to Miss Mapple and Nancy Beef to give an insight into the final steps, which involve separation, and the subsequent nitrogen and protein determination. Also, find out what Lieutenant Cornlumbo has been up to all this time? And what the special prize is to be awarded to one of the lucky detectives?
The detectives had all gathered for the second part of the in depth Kjeldahl workshop. Shallot Holmes and Eggcule Poirot, seemed very relaxed having already given their talks in the previous workshop. Miss Mapple and Nancy Drew were looking less relaxed but eager to proceed. Lieutenant Cornlumbo, was sat quietly in the corner, still looking mischievous and very self-satisfied.
Miss Mapple got everyone’s attention and proceeded to describe the separation step. She explained that after the digestion step explained by Eggcule Poirot, the sample is allowed to cool to room temperature. At this point, the next process in the Kjeldahl method involved the acidic digestion mixture being diluted with distilled water, and the sample tube getting transferred to a distillation unit. The separation step involved the solution being alkalized with sodium hydroxide to liberate the ammonia, which is then distilled into an acidic receiver solution by steam distillation. Ideally, the distillation should continue until 99.5% of the ammonia is recovered in the receiving vessel.
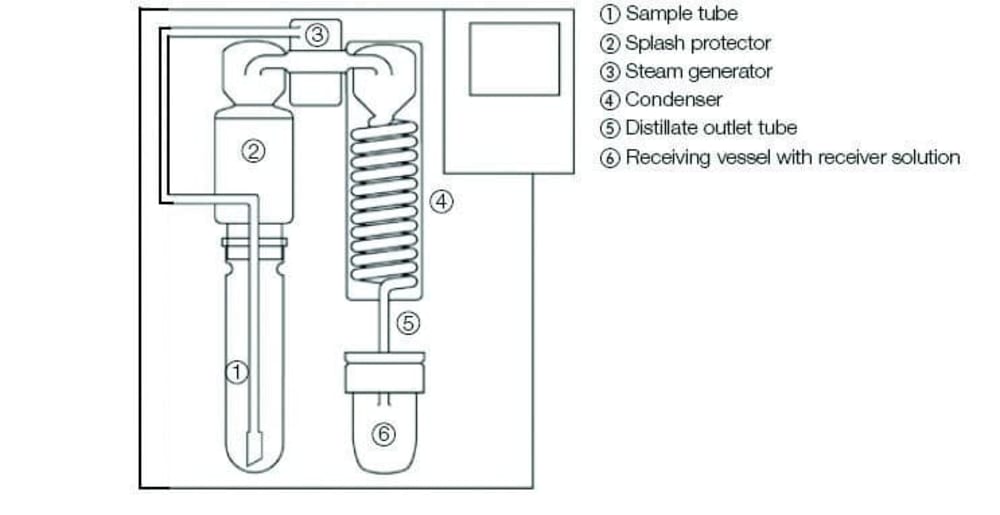
“In a steam distillation unit, the steam generator (3) produces steam which is introduced into the sample tube (1) to drive out volatiles, in this case, the ammonia. After condensation in the condenser (4) the distillate is collected in a receiving solution (6). An important consideration for our clients is that method parameters must be varied according to how the final step is performed. For boric acid titration, an aqueous boric acid solution of 2 or 4 % concentration is required, whereas, for back titration, the absorbing solution is a precisely dosed volume of H2SO4 standard solution.”
Miss Maple pointed out the things that clients should be aware of. One issue is that samples left to cool for too long can crystalize. Manual distillations may lead to low results for nitrogen if the solids are not dissolved. Crystallization can be avoided by:
– Adding 1 – 2 mL more sulfuric acid for the digestion.
– Diluting samples with 10 – 20 mL of distilled water after cooling.
Another issue is splashing due to overheating when hot steam enters the mixture already heated by the previous reaction; this can be avoided by setting a reaction time on the distillation unit after the alkalization of the acid.
“Lastly, the starting volume of distillation is critical. If the starting volume is too large, there is a risk of carryover of sample solution into the condenser and receiver. A general rule applies here, for distillation in 300 mL sample tubes, a starting volume not larger than 200 mL, and in 500 mL tubes, a volume of 300 mL should not be exceeded. If all these things are taken into consideration then the clients should have performed an effective separation and be all set for the final step” Concluded Miss Mapple creating the perfect segue for Nancy Beef.
Nancy Beef took to the stage and thanked Miss Mapple for her thorough presentation and went on to explain the final process of the Kjeldahl method. The quantification of ammonia by means of titration. “The pH in the acidic receiver solution will rise with the addition of ammonia and water. The nitrogen and protein content are then determined through titration of the borate complex.”
Nancy explained that the choice of method for the final step would often be dictated by regulations surrounding quality control. “Two techniques can be applied for the detection of pH: potentiometric and colorimetric titration. Potentiometric titration measures the pH directly by means of the potential of acidic hydronium ions in the solution with a pH electrode, whereas, colorimetric titration measures pH-dependent color changes with a colorimetric sensor that measures light absorption at a suitable wavelength”.
“Two types of titration are generally used for protein and nitrogen determination: Boric acid titration and back titration. Boric acid titration is the most commonly used method as it involves direct detection and can be automated without additional equipment, something our clients always appreciate.” Said Nancy. She went on to explain how the concentration of ammonium ions is captured into a receiving solution of boric acid, and the subsequent endpoint titration is performed with a stronger acidic titration solution. An advantage of this method is that it does not require an accurate dosage of boric acid.
“The alternative method is back titration, which requires a precise dosage of a standardized acid in the receiving vessel. After the ammonia has been collected, the excess acid is titrated with a basic titration solution at pH 7.00. Regulations may, however, dictate that a different endpoint pH has to be chosen. Back titration is often the procedure of choice if the use of boric acid must be avoided.”
Nancy then explained the things that their clients must be weary of, such as, air bubbles in the burette or titration tubing that could cause a false titrant consumption reading, yielding high results. “Incorrect results could also be caused by using the wrong titrant, having air bubbles in the colorimetric sensor – which interfere with its light beam, the wrong ratio of indicator, calculation errors, or a defective or badly calibrated electrode.” Concluded Nancy Beef.
Shallot Holmes thanked each of the detectives for their thorough presentations and told them how excited he was to share their findings. He was convinced that the knowledge and tips provided by the detectives would assist method development and help ensure that several potential problems would be avoided.
“Just one more thing,” said Lieutenant Cornlumbo, and by the looks on the detective’s faces, they were keen to find out what he had to say. What had Lieutenant Cornlumbo been tasked with? Who would get the special prize?!
“I have a question for you detectives, highest or lowest?”. Said Cornlumbo. The detectives looked very confused. “It’s a simple choice, we’ll take a vote, and that will determine who gets the special prize”. They took a vote, and the majority went for ‘highest’. “Ok then, highest it is”.
Lieutenant Cornlumbo had been tasked with determining the protein content of each of the detectives! And the clear winner by far was Nancy Beef, with a protein content of 22 %. Shallot Holmes presented Nancy with a bottle of fine Red Wine that he said would complement her very well indeed.
