From Flavors to Fuel: Free Fatty Acid Determination in Flour and Brewers Spent Grain
Chapter 59
? Case overview: The detectives delve into the fascinating world of Free Fatty Acids (FFAs) and help a client extract them using Soxhlet extraction. Understanding and determining FFAs is essential to ensure quality control and optimize production processes.
How can you Perfect the Art of Making Sourdough Bread?
Nancy Beef has brought the detectives some sourdough bread that she baked. Cornlumbo immediately starts to wolf down the bread with lashings of butter. “You know, there’s something magical about sourdough. It’s the wild fermentation that creates complex flavors and textures,” says Miss Mapple, watching Cornlumbo help himself to another slice. “It took me ages to get the large air bubbles; I researched everything, from the fermentation to the development of gluten,” says Nancy. She then explained how the bacteria in the sourdough starter consumed sugars in the dough, producing carbon dioxide as a by-product, and that the strength and elasticity of the gluten network determined how well the dough could trap and hold gas. She explains how she finally got the hydration and timing right and perfected the baking conditions. Holmes asks if Nancy had considered how oils and fats impacted the final product. “Oh, you mean like how olive oil makes bread softer and more aromatic but also slightly denser because the fats encase the flour proteins, reducing their ability to form strong gluten networks,” says Nancy. “Exactly,” says Holmes. Cornlumbo then says that adding butter makes bread more yummy. “Less technical, Cornlumbo, but true all the same,” says Holmes. Impressed with Nancy’s practical working knowledge of fats, Holmes asks if she could assist some clients in determining Free Fatty Acid (FFA) content in flour and brewer’s spent grain. Nancy is pleased to help and cannot wait to further improve her knowledge of flour, which will prove invaluable to her baking.
What are Free Fatty Acids?
Before Nancy sees the client, Holmes gives the detectives an overview of FFAs. He explains that they are vital energy sources for most body tissues and are classified according to their aliphatic tail length. The word aliphatic is derived from the Greek aleiphar, meaning fat or oil, which is fitting because many oils and fats are composed of aliphatic compounds. There are short (fewer than 6 carbon atoms), medium (6 – 12 carbons), and long-chain (12 or more) fatty acids. They are carboxylic acids comprising a hydrocarbon chain and a terminal carboxyl group. Unlike triglycerides, FFAs are unattached to other molecules and, hence free. They are produced when triglycerides are hydrolyzed, and the ester bonds that link the glycerol backbone to the fatty acid chains are broken. This reaction typically requires water and may be catalyzed by enzymes like lipases or chemicals like acids or bases.
1 Triglyceride + 3 H2O 1 Glycerol + 3 Free Fatty Acids
FFAs can be saturated or unsaturated based on the presence or absence of double bonds in their hydrocarbon chain. The chain's length and saturation influence the fatty acid's properties, including its melting point and function. Saturated fatty acids have a straight chain that allows for tight packing, making fats more solid, like butter. Unsaturated fatty acids have kinks in their structure due to the double bonds, making it harder to pack closely together, leading to a liquid state as the melting point is below room temperature. Holmes asks Nancy if she knows the importance of FFAs in flour and brewers' spent grain (BSG) and why the client may be interested in extracting them.
Why is Free Fatty Acid Content in Flour and Brewers’ Spent Grain Important?
Nancy knew that the client would need to know the FFA content in flour as it relates to the nutritional quality and shelf life of the flour. FFAs would increase during storage as the fats and oils break down due to hydrolytic and oxidative rancidity. Oxidized FFAs smell rancid and may be a key factor for quality control for the client. Nancy admits that she doesn’t know much about brewers' spent grain, but Eggcule, who has previously helped clients determine analytes in beer, gives a brief summary.
“When making beer, malted grains are soaked in water and heated to convert the starches into fermentable sugars. This liquid, now rich in sugars, is called the wort, which is drained off and boiled with hops before fermentation with yeast to produce beer. The solid residue left after the wort is drained is the brewers’ spent grain,” says Eggcule. He explains that FFAs in BSG, like flour, can influence the nutritional quality and shelf life; however, FFAs can also have a positive impact depending on the client's application. The FFAs in BSG can be converted to biogas through anaerobic digestion or into methane for renewable energy if converted by specific microorganisms. Therefore, spent grain with a high FFA content might be an attractive substrate for biogas production.
How do you Perform Free Fatty Acid Extraction and Determination?
Nancy heads to see the client and helps them perform an extraction to determine the content of their samples using the following method.
Weighing
The first step was for the client to add sodium sulfate (Na2SO4) to a cellulose thimble, weigh a known amount of flour, and record its weight.
Soxhlet Extraction
The extraction is then performed using the Soxhlet Extraction method. First, you must weigh dry and clean beakers. The cellulose thimbles with the samples are then inserted into the Soxhlet apparatus, and the level sensors are adjusted to match the heights of the samples. A suitable solvent is added (e.g., hexane or petroleum ether) to the beakers and placed into the Soxhlet apparatus. The beaker is heated, causing the solvent to evaporate, condenses at the condenser, and drops into the extraction chamber.. The solvent will continuously siphon through the sample, extracting the fat from the flour. Continue the extraction until the solvent from the siphon tube is clear, indicating the complete extraction of fats.
Drying and Weighing of the Extract
Once the extraction is complete, remove the beaker containing the fat and place it into a drying oven until a consistent weight is achieved. After drying, you’ll be left with the extracted fat, which needs to be weighed and recorded.
Titration of FFA
Dissolve the extracted fat in a known volume of neutral alcohol (like ethanol) and add a few drops of a phenolphthalein indicator to this solution. Titrate the solution with a standard alkaline solution, typically sodium hydroxide (NaOH). The alkaline solution will neutralize the FFAs. Continue the titration until a pink color persists for about 30 seconds, which indicates the endpoint. Record the amount of NaOH used.
Calculation of the Fat Content and FFA Content
Nancy then provides the client with three equations for calculating FFA content. The first is a simple equation to calculate the fat percentage in the sample. The second equation was for flour samples and expresses the results as the quantity of sodium hydroxide needed to neutralize the fat of 100 g of dry matter. The third equation articulates the percentage of FFA in brewers’ spent grain, expressed in terms of oleic acid. Oleic acid is a common monounsaturated fat and is a standard reference for such calculations.
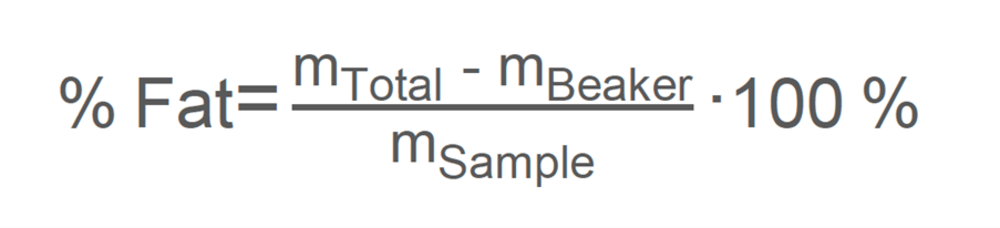
% Fat: Percentage of fat in the sample.
mTotal: The combined weight of the beaker and the extracted fat [g].
mBeaker: The weight of the empty beaker [g].
mSample: The weight of the original sample you started with [g].
Equation Two
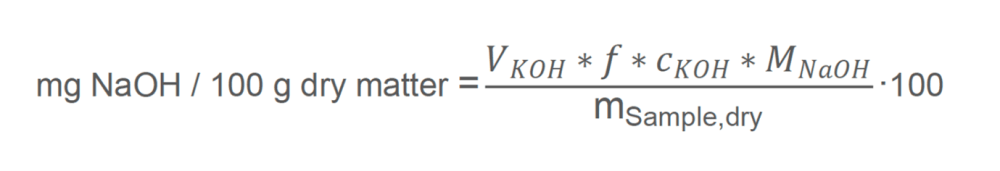
mg NaOH / 100 g dry matter: the amount of sodium hydroxide (measured in milligrams) required to neutralize the FFAs present in 100 g dry matter. VKOH: Titrant consumption [mL]. It indicates the volume of KOH solution used during titration.
f: correction factor [ - ]. This is to account for any experimental anomalies or systematic errors, ensuring more accurate results.
cKOH: The concentration of the titrant, KOH = 0.1 mol/L
MNaOH: Molecular weight of NaOH [g/mol]. Sodium hydroxide has a molecular weight of approximately 40 g/mol. This value converts the amount of substance from moles to grams.
mSample,dry: The dry sample weight [g].
100: The factor used to express the result per 100 g dry matter for easier interpretation and comparison.
Equation Three
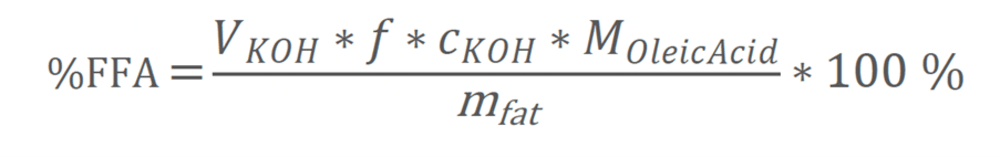
%FFA: The percentage of free fatty acid per gram of fat expressed in oleic acid.
VKOH: Titrant consumption [mL]. f: correction factor [ - ].
cKOH: concentration of titrant, KOH = 0.1 mol/L.
MOleicAcid: Molecular weight of Oleic Acid [g/mol]. Oleic acid has a molecular weight of about 282.47 g/mol.
mfat: The total weight of extracted fat from the sample [g]. The client was very happy using Nancy's method as they obtained the following data that proved highly accurate with very low standard deviations.
Sample | Mean value [mg / 100 g] | RSD | Assigned Value |
Flour sample 173-0102 | 32.15 | 0.83% | 37 ± 5 |
Flour sample 174-0102 | 48.30 | 1.05% | 44 ± 5 |
Flour sample 175-0102 | 35.80 | 0.82% | 43 ± 5 |
Flour sample 176-0102 | 39.88 | 0.78% | 45 ± 5 |
Brewer’s Grain 1 | 13.14% | 0.55% | 11% – 16.3% |
Brewer’s Grain 2 | 16.91% | 0.56% | 11% – 16.3%
|
Determined FFA content in flour and BSG (RSD: Relative Standard Deviation), n = 3
And to thank Nancy, the client said she could always come to them for free flour for her baking whenever she liked. Nancy was ecstatic and immediately went home to bake a cake for the detectives as a treat.
