Kjeldahl Masterclass: How to reduce costs and optimize the process
? Workshop overview: Holmes gives the detectives a masterclass on Kjeldahl digestion, a method for the qualitative determination of nitrogen contained in organic compounds. Many of the detective’s clients rely on the method for analyzing proteins as it is internationally recognized and the standard against which other methods are compared. Holmes wants to focus on using the latest instruments to optimize the process and reduce the chemicals required, as it will help their clients save money and benefit the environment.
Nancy Beef has just returned from her expedition to the Pacific Northwest, where she successfully solved a case for a client determined to ensure the freshness of the fish they sold to high-end restaurants. She is showing the detectives pictures of the delicious salmon meal she was given as a reward for solving the case. Cornlumbo cannot believe what he is seeing. The food looked like a work of art, and from what Nancy said, it tasted even better. “I can hear your stomach rumbling from here,” says Miss Mapple. Holmes then enters the office, congratulates Nancy for her hard work solving the case, and tells the detectives that he has prepared a masterclass for them. The detectives are excited to find out what Shallot Holmes has prepared for them as they are usually the ones who must prepare workshops for Holmes. They all gather around and prepare to take notes.
“As you all know, the Kjeldahl method has been used by chemists since its development 140 years ago and has been improved significantly over the years. Improvements and optimizations are still being made that make the process more efficient and environmentally friendly. Kjeldahl is so important to our clients that you previously prepared two workshops explaining the method in detail, from sample preparation and digestion to steam distillation and titration. So, today detectives, I would like to go over the most important points to consider for optimization and explain how the latest sensors and technologies can make the process better than ever before.” Says Holmes.
Holmes then explains that the first thing that needs to be considered before the process begins is the sample and, specifically, the size of the sample. If you can determine the minimum sample size required for accurate analysis, you minimize the reagents and chemicals required for each analysis – less sulfuric acid, catalyst, NaOH, and titrant are needed. The best way to maintain high precision is to aim for a sample size of 10 mg of N in the sample tube. The amount of nitrogen needs to be high enough to measure accurately but small enough to fall within the linear range of the titration method. For certain inhomogeneous samples, such as soil, you may require a larger sample to be representative. When weighing samples, you need to ensure that the weight is not too low for the balance, as weighing errors can occur that will affect the accuracy of the results. Therefore, optimizing the sample size for the Kjeldahl method involves striking a balance between accuracy, precision, representativeness, and resource efficiency.
“Optimizing the sample size for the Kjeldahl method involves striking a balance between accuracy, precision, representativeness, and resource efficiency.”
When a smaller sample size is used, other aspects of the method (such as the digestion and titration stages) must also be optimized to maintain high precision and accuracy. One development in modern instruments is the addition of sensors that monitor the process and accurately control dosage to minimize wastage. Once a sample has been prepared, it undergoes catalyzed mineralization, transforming the organic nitrogen into ammonium sulfate using a boiling mixture of sulfuric acid and sulfate salts. Next, the solution is alkalized, liberating the ammonia, which is then steam-distilled and quantified through titration. During the sample’s mineralization, sulfuric acid is consumed to produce carbon dioxide, water, and sulfur dioxide. A high level of organic matter in a sample means more sulfuric acid is used, leaving less acid in the sample tube. Traditional Kjeldahl distillation involves a specific dosage of sodium hydroxide solution designed to accommodate the highest possible quantity of sulfuric acid after mineralization. Typically, it’s given in excess to ensure complete alkalinization, which is crucial for liberating the ammonia and achieving accurate protein content measurement. Although this process ensures the completion of the process, it is also very wasteful, which is why the Reaction Detection Sensor (RDS) was created.
When Kjeldahl distillation is conducted with the RDS, the sensor detects the end of the alkalinization and promptly halts the sodium hydroxide dosage. Consequently, samples with less sulfuric acid receive less sodium hydroxide. This principle is depicted in the Figure below, which illustrates the functionality of the RDS technology.
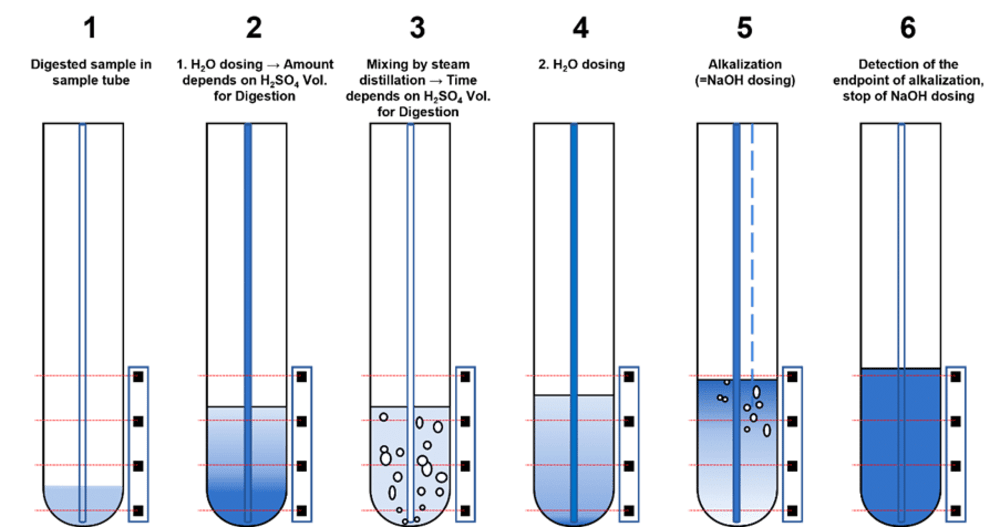
Optimizing the Kjeldahl process by employing the Reaction Detection Sensor brings numerous benefits. Primarily, it saves sodium hydroxide (NaOH), as it immediately halts alkalinization upon reaching a pH of ≤12. Furthermore, defining distillation parameters for the water and sodium hydroxide volumes is not necessary. Users need only input the volume of concentrated sulfuric acid used in the digestion stage. The system also accommodates slightly crystallized samples due to its automated pre-conditioning feature.
Upon the initiation of a process, the RDS starts with the pre-conditioning of the sample. In this stage, water is added, and the sample is homogenized via steam distillation. This pre-conditioning is essential in reducing the vigor of the acid-base reaction during the ensuing alkalization. The added water mixes with the sample during this process, ensuring that the acid-base reaction occurs rapidly enough to be detectable, regardless of the sample’s condition following the digestion step. The RDS uses a sensor to optically monitor the acid-base reaction in the sample tube during alkalinization. The sensor employs an algorithm that identifies the moment when the sample has been sufficiently dosed with NaOH. At this point, it triggers the dosing to stop. This efficient and precise control mechanism contributes significantly to the optimization of the Kjeldahl process.
The following table provides a comparative overview of Kjeldahl distillation parameters or steps, contrasting the processes with and without the use of a Reaction Detection Sensor. When using traditional Kjeldahl distillation without the RDS, three parameters must be defined: the volume of water (H2O), sodium hydroxide (NaOH), and the reaction time. However, when employing the RDS, only one parameter needs to be specified: the volume of sulfuric acid (H2SO4) used for digestion. The system then uses this information to perform sample pre-conditioning and water dosage automatically.
| Distillation parameter/step | Without RDS | With RDS |
| Sample pre-conditioning | not performed | performed |
| H2SO4 Vol. for Digestion | not required | required (mL) |
| H2O Volume | required (mL) | not required (automatically dosed) |
| NaOH Volume | required (mL) | not required (dosage stops automatically after detection) |
| Reaction Time | required (s) | not required |
“To conclude, I feel that many of our clients could benefit from the optimizations I have outlined as they will save money and time and also help reduce their environmental footprint, a win win win situation,” says Holmes.
The detectives, who have been making notes throughout, are grateful for the insight provided by Shallot Holmes and look forward to sharing their knowledge with future clients. Holmes is glad the detectives could take a break from their casework and offers to take them all out to dinner. All the detectives enjoy a full three-course meal, apart from Cornlumbo, who says he could eat more and is considering eating an additional cheese course. Miss Mapple says, “I think we need a Reaction Detection Sensor for Cornlumbo’s stomach to let him know when to stop the digestion process!” much to the amusement of the other detectives.
