Mastering Kjeldahl: Nitrogen Determination in Feed Products

The detectives are feeling pleased about their work producing a document for their clients on improving sustainability in the lab. As Cornlumbo celebrates by diving headfirst into a tub of Lickety Splits finest ice cream, Miss Mapple ponders the sustainability of Cornlumbo. “Have you heard of the term ‘treatwise’ Cornlumbo?” asks Miss Mapple. “Treatwise? Is that a method to determine which order to eat sweets to avoid indigestion?” asks Cornlumbo. “No! It’s an initiative from confectionary makers to help families have a balanced approach to treats,” explains Miss Mapple. “Oh, well, my approach to treats is always balanced; I eat them all equally!” declares Cornlumbo as Miss Mapple puts her head in her hands in despair. “For all the work we do helping clients determine the nutritional composition of their products, you’d think you may actually look at labels once in a while and try to balance your diet,” says Eggcule Poirot.
“Cornlumbo is a fully grown adult, or perhaps overgrown… either way, he is able to decide for himself what he puts in his body. But can you tell me detectives, who doesn’t have the same choice and cannot make use of the nutritional labels Eggcule speaks of?” asks Shallot Holmes. The detectives look a little confused, thinking perhaps Holmes is talking in riddles. After a short while, Holmes puts the detectives out of their misery. “Animals,” says Holmes before explaining the details of their latest case.
What is the role of food and feed regulatory bodies?
“As Eggcule pointed out, we have helped many clients with various methods of determination to ensure they comply with labeling regulations. Various regulatory bodies exist worldwide to ensure that the methods of determination provide accurate results, such as the U.K. Food Standards Agency (FSA) and the European Food Safety Authority (EFSA). These organizations work with local authorities that enforce the guidelines by conducting inspections, serving prohibition notices, or prosecuting businesses. However, the role of these regulatory bodies is not just enforcement; their primary mission is to provide impartial scientific advice that serves as the basis for laws and regulations to protect consumers, including livestock. And that’s where we come in. We have been asked to put together some guidance notes and best practices for protein determination for the feed industry,” explains Holmes.
Why analyze the nitrogen and protein content of animal feed?
“Listing nutrients for animal feed seems pointless if the animals can’t read the labels,” says Cornlumbo. “There are many reasons to analyze these things, especially for feed. The overall protein content is important for the payment of raw materials; it’s a signifier of basic nutrients, affects the calorific value, and is also a quality parameter that influences the production process,” says Miss Mapple. “You’re quite right, but what I’d like us to focus on is the most compliant method of protein determination, the Kjeldahl method,” says Holmes before setting the detectives the task of providing scientific advice about the Kjeldahl method for protein determination.
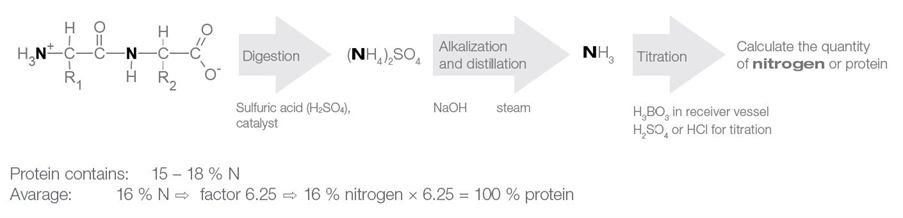
What is the Kjeldahl method?
The Kjeldahl method determines the protein content of a sample by means of determining the amount of nitrogen present. The amount of nitrogen can be used to determine protein content because proteins are primarily composed of amino acids that contain nitrogen. Once the amount of nitrogen has been determined, it is multiplied by a conversion factor of around 6.25, based on the assumption that proteins contain approximately 16% nitrogen by weight.
The process involves four main steps:
- Sample Preparation
- Digestion
- Alkalization and Distillation
- Titration
What are the detective’s top Kjeldahl tips?
Sample Preparation
The guidelines set out by organizations such as the FSA and the EFSA aim to ensure accuracy and repeatability. Therefore, sample homogeneity is of particular importance to avoid variability. Inhomogeneous samples would increase the likelihood of standard deviations in repeated determinations. The accurate weighing of samples and selecting tubes of the right size are critical. For Kjeldahl, three sizes are used depending on the nitrogen content of the sample: Macro, semi-micro (standard), and micro:
- Macro Kjeldahl: For 10 – 30 mg N, use 300 or 500 mL sample tubes
- Semi-micro Kjeldahl: For 0.1 – 3 mg N, use 300 mL sample tubes
- Micro Kjeldahl: For 1 – 15 mg N, use 100 mL sample tubes
Top tip:
- Foam formation is a common problem associated with Kjeldahl digestions, especially when larger sample volumes are used. Anti-foaming agents can help here, such as adding stearic acid or a dedicated antifoaming tablet.
Digestion
(CHNO) + H2SO4 à CO2 + SO2 + H2O + NH4+
This is where the organically bonded nitrogen is converted into ammonium ions, and organic carbon and hydrogen form carbon dioxide and water. The carbonization step involves the oxidation of organic material with sulfuric acid whereby the formation of water can be observed when it condenses at cooler parts of the glassware.
Top Tips:
- Optimal digestion conditions are achieved when the condensation zone remains 5 cm below the constriction of the sample tube.
- After the digestion has led to a clear liquid, a further 30 minutes of digestion time can be added to allow complete mineralization.
- Carbonization starts at room temperature and increases at higher temperatures. The addition of salts and the use of catalysts or hydrogen peroxide allows for shorter digestion times.
- Water-containing samples can cause strong foam formation and sputtering. For such samples, start with a lower temperature and increase slowly.
Alkalization and Distillation
Neutralization/Alkalization: H2SO4 + 2 NaOH à 2 Na+ + SO42- + 2 H2O
Distillation: NH4+ + OH- ⇌ NH3 (gas) + H2O
After digestion, the sample must be allowed to cool to room temperature before diluting the acidic mixture with distilled water and alkalizing it with NaOH. The ammonia is then steam-distilled into an acidic receiver solution. The solvated ammonium ions (NH4+) produce ammonia gas (NH3) by reacting with hydroxyl ions (OH-) of excess sodium hydroxide (NaOH). During steam distillation, ammonia is separated from the sample and condensed with the water in the receiving vessel.
Top Tips:
- The sample must be diluted to avoid splashing caused by boiling, which is induced by the heat of the reaction of the concentrated acid and base being mixed.
- If digested samples cannot be processed directly after cooling, crystallization may occur, skewing the results if the solids are not dissolved. To avoid crystallization, use 1 – 2 mL more sulfuric acid for the digestion process.
Titration
Receiver: B(OH)3 + NH3+ H2O ⇌ NH4+ + B(OH)4-
Titration: B(OH)4- + HX ⇌ X- + B(OH)3 + H2O
The pH in the acidic receiver solution will rise upon the addition of ammonia, and the nitrogen and protein content will then be determined by titration of the borate complex.
Titrations may be carried out manually using a burette and an appropriate pH indicator to indicate the endpoint of pH 4.65 or by using a dedicated titrator to read the volume of consumed acid from the display. Some advanced distillation units have a built-in titrator, and the process can be automated with the results calculated by software on the instrument.
Top Tips:
- Certain official methods require colorimetric rather than potentiometric titration. Potentiometric titration is based on measuring electrical voltage using a pH electrode. Colorimetric titrations involve using an indicator to measure pH-dependent color changes that measure the absorbance of light.
- Watch out for air bubbles, as they influence the titrant consumption reading and skew the results, giving higher readings.
