Saying yes to non-protein nitrogen determination in milk

Chapter 23
? Case overview: Shallot Holmes tries to dissolve the boredom that has settled over his team by taking on a case of non-protein nitrogen determination in milk samples. Will their chosen reference method enable them to perform reliable determinations that correspond to literature values, or will they add disappointment to their boredom? Read on to find out.
Shallot Holmes rummages through the pile of cases on his desk. The other detectives watch him silently. Nancy Beef is trying to stifle a yawn. Eggcule Poirot is nervously tapping his finger on the side arms of his chair. Lieutenant Cornlumbo is secretly eyeing the clock. Only Miss Mapple is sitting contently, quietly munching on a piece of apple strudel (oh yes!).
Shallot Holmes pulls out a case file with a grunt. He looks at the faces of his bored colleagues and says that he would like to lead the case. He admits the topic might not infuse them with excitement, but he will do his best to make it as interesting as possible.
The client has a straight-forward request. He works as a quality control manager on a dairy farm, and he has been tasked with finding a reference method for non-protein nitrogen determination in milk as per ISO 8968-4:2001(E).
The detectives are already visibly perking up. They are looking at each other wondering what on earth is non-protein nitrogen!
Shallot Holmes can read the curiosity on their faces and begins to explain. Non-protein nitrogen is a normal part of milk. The non-protein nitrogen (NPN) fraction is composed of urea and other low-molecular weight nitrogen containing compounds such as creatine and creatinine.
Approximately 50% of non-protein nitrogen in milk is urea and variations in NPN is attributed primarily to variation in urea content.
Non-protein nitrogen has little nutritional value and does not contribute to cheese yield. Nonetheless, determination of NPN in milk is a routine analysis needed in quality control.
Lieutenant Cornlumbo, who is a fan of all things unconventional, is listening intently. He asks the group how this strange non-protein nitrogen is typically measured.
Shallot Holmes gives the team a brief overview of one reference method used to determine non-protein nitrogen content, the Kjeldahl method. The steps include:
- Precipitating protein from a test portion by adding trichloroacetic acid solution
- Digesting the filtrate with sulfuric acid to convert nitrogen into ammonium sulfate
- Alkalizing with sodium hydroxide to convert ammonium sulfate to ammonia
- Distilling the sample into a boric acid receiver by steam distillation
- Titrating with hydrochloric acid solution
- Calculating the non-protein nitrogen content by using the volume from the hydrochloric acid and the weight from the sample, trichloroacetic acid solution and filtrate.
The detectives collectively decide to use this method and to demonstrate its validity to the customer by using two different samples of grocery-store-bought milk:
- Whole milk UHT, declared protein content 3.2 g/100 ml
- Partial skimmed milk UHT, declared protein content 3.2 g/100 ml
Then they need to find the literature values of the expected non-protein nitrogen content in their milk samples. It turns out that the amount of NPN in milk varies naturally. In some literature, they find values that range between 0.019 to 0.039%, in other references, they find non-protein nitrogen concentrations between 5 to 6.5% of the total nitrogen.
The detectives then homogenized the milk samples by shaking and digested them following similar steps of the Kjeldahl method as in their previous cases.
The team went on to set up their Kjeldahl Digester as follows:
| Step | Temperature (°C) | Time (min) |
|---|---|---|
| Preheat | 350 | - |
| 1 | 550 | 35 |
| 2 | 490 | 45 |
| Cooling | - | 30 |
After the digestion was completed, the detectives proceeded to distillation and titration by setting up their Kjeldahl system with the following parameters:
| Kjeldahl system | Titrator | ||
|---|---|---|---|
| Water | 80 mL | Titration Solution | HCl 0.01 mol / L |
| NaOH | 80 mL | Internal Method | pH fast strong |
| Boric acid 2% | 50 mL | Endpoint | pH 4.6 |
| Reaction Time | 5 s | ||
| Steam Power | 100% | ||
| Dist. Time | 240 s | ||
| Titration Start | 240 s | ||
| Titration Type | Boric Acid | ||
| Stirrer Sp. Dist. | 5 | ||
| Stirrer Sp. Titr. | 7 |
After the process was finished for their samples, Shallot Holmes calculated the non-protein nitrogen content as a percentage of nitrogen with the following equation:
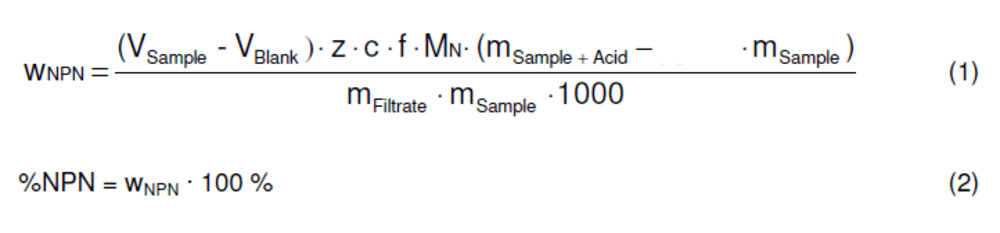
wNPN : weight fraction of non protein nitrogen
VSample : amount of titrant for the sample [ml]
VBlank : mean amount of titrant for the blank [ml]
z : molar valence factor (1 for HCl, 2 for H2SO4)
c : titrant concentration [mol/l]
f : titrant factor (for commercial solutions normally 1.000)
MN : molecular weight of nitrogen (14.007 g/mol)
mSample : sample weight [g]
mFiltrate : filtrate weight [g]
mSample + Acid : sample plus trichloroacetic acid weight [g]
%NPN : percentage of weight of non protein nitrogen
0.065 : is the multiplication factor, based on the assumption that milk
contains a mass fraction of about 3.5 % fat and 3.0 % true protein
(thus 0.035 + 0.030 = 0.065) / for partial skimmed milk with 2.7 % fat
the factor is 0.057.
The detectives have long forgotten their boredom. They are nervously jumping from foot to foot, trying to peek over Shallot Holmes’ shoulder and see the results. Shallot Holmes presents them the final data:
| NPN Content (%) | |
|---|---|
| Whole milk | 0.025 (0.85%) |
| Part. skimmed milk | 0.025 (0.63%) |
The detectives let out little yelps of victory. Even this strange non-protein nitrogen was detectable with Kjeldahl. The client will certainly be happy with the reference method they just tested out for him! After all, they were easily able to determine non-protein nitrogen content in milk with reliable and reproducible results with low relative standard deviations with values that correspond to those found in literature.
Now that the detectives have left their boredom behind, they are ready for even more milky activities. As night falls, they settle in on their terrace, wrapped in cozy blankets, holding warm mugs full of milky hot chocolate and gaze at the sky, enjoying the Milky Way. They quietly enjoy each other’s company and empty their heads of protein and non-protein nitrogen alike, ready for a fresh start tomorrow.
