Staying Fresh: Uncovering the Secrets of Sulfur Dioxide Analysis
As the sun peeked over the horizon, casting a warm golden hue over the landscape, Vinny the Vintner bounded toward his wine cellar. He had been awake since dawn, as today was not just another day; it was the day he would finally witness the fruits of his labor. The winery was his life’s work, a dream he had nurtured like the vines in his vineyard.
With a spring in his step, he took the key to the heavy oak door from his pocket and entered the cellar. His heart filled with a mixture of hope and pride as he looked upon the fruits of his labor. Rows and rows of wine bottles stood to attention. Vinnie approached the racks of wine, picked up a bottle, and held it to the light. The wine, a rich, deep red, was the culmination of years of toil, learning, and passion. A smile crept across his face as he imagined wine lovers savoring each drop.
But then, suddenly, a soft pop echoed through the cellar, faint but unmistakable. Vinnie’s smile faltered. He turned his head just in time to see a cork rocketing off a bottle at the far end of the shelf. Confusion wrinkled his brow, but before he could register what had happened, another pop, then another. Like a cacophony of tiny cannons, corks began to fly off bottles, each one a bullet of disbelief striking Vinnie’s heart.
Wine spouted like miniature geysers, turning his pristine cellar into a scene of utter chaos. Vinne stood frozen, his dream unraveling before his eyes to a mocking melody of popping corks.
As the last cork flew and the final droplet of wine splattered onto the stone floor, Vinnes vineyard, once a sanctuary of his aspirations, lay drenched in the remnants of a dream gone awry.
? Case overview: A wine-producing client is having an issue with the longevity of the wine they produce. The detectives have been called in to help them master the addition of sulfites to ensure consistency and longevity.
In the detectives’ office, the detectives were still very enthused about their health and discussed the progress of their New Year’s resolutions. The topic of conversation had shifted from nutrition and exercise to longevity and prolonging life. Cornlumbo, still obsessed with the progress of AI, believed that soon, AI would find a way to enable the detectives to extend their lives considerably or even live forever. Shallot listened as the detectives talked, and he waited for them to finish their breakfast before craftily relating their topic of conversation, as he often did, to the latest case he had for them.
“I believe it was Richard Feynman who said that ‘in wine is found the great generalization: all life is fermentation. Nobody can discover the chemistry of wine without discovering, as did Louis Pasteur, the cause of much disease’ and seeing as you are all so keen on extending life, I hope you can help our latest client who is having an issue with the shelf life of his wine,” says Shallot Holmes. Holmes explained the client’s predicament and the case of the popping corks.
Eggcule Poirot, who had experience in helping determine analytes in beer and wine, prided himself on his knowledge of the fermentation process. It was immediately clear to him that secondary fermentation could have caused the corks to pop off. “Secondary fermentation occurs when residual sugar in the wine undergoes fermentation after bottling. Yeast present in the wine consumes the sugar and produces CO2 as a byproduct. In a sealed bottle, the CO2 has nowhere to escape and builds up pressure. Eventually, this pressure can become so great that it forces the cork from the bottle,” says Eggcule. Holmes is impressed with Eggcule’s knowledge and asks him how the client could prevent this from happening.
Secondary fermentation occurs when residual sugar in the wine undergoes fermentation after bottling. Yeast present in the wine consumes the sugar and produces CO2 as a byproduct
Eggcule suggests the addition of sulfites as they have preservative and antioxidant properties. He explains how they inhibit the growth of yeasts and bacteria, preventing them from consuming residual sugars. Holmes agrees that this will indeed help the client, but he also knows that Vinnie is very proud of his wine, and he will be very reluctant to do anything that may taint the quality of his wine. The detectives are aware of the importance of quality control in the Food and Feed sector, and Eggcule understands that SO2 levels can impact the taste, aroma, and overall quality of food and beverages. “Accurate SO2 determination is going to be critical for the vintner’s production and also for labeling requirements,” says Eggcule.
Holmes asks the detectives for an efficient method of sulfur dioxide analysis. Miss Mapple describes the modified Monnier-Williams method, which is often used for food and beverage applications. She explains that the modified technique is faster than the classical technique as it doesn’t require the nitrogen purge step. The Monnier-Williams method is an effective way of determining the amount of steam-volatile sulfur dioxide (SO2) and sulfite (SO32-) and involves distilling the sample with an acid mixture which converts sulfite ions to steam-volatile sulfur dioxide:
SO32- + 2 H3O+ →SO2 + 3 H2O.
The method is based on pH titration, which can be performed directly on certain instruments. Miss Mapple describes the steps that are required:
- Place the sample with the acid mixture in the sample tube and start steam distillation with 3% H2O2 solution in the receiving vessel.
- The released SO2 is absorbed in the H2O2 solution pre-dosed in the receiver, and the following (simplified) reaction takes place to yield sulfuric acid. SO2 + H2O2 →H2SO4
- The receiving solution is then titrated with a standard NaOH titration with a pH electrode to quantify the amount of formed sulfuric acid.
The following calculations are used to determine the mass and concentration in the sample:
Mass of SO2:
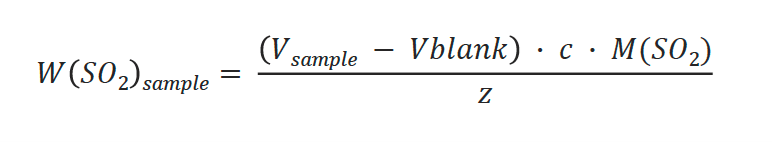
Concentration of SO2:
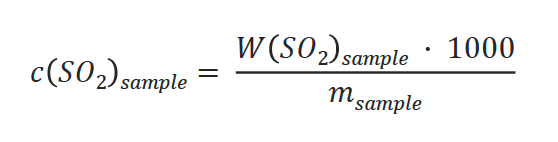
- W(SO2): Weight of SO2 [mg SO2]
- M(SO2): Molar mass SO2 = 64.0648 [g/mol]
- Vblank: Titrant consumption for blank [mL]
- c: Concentration of NaOH (titrant) [mol/L]
- z: valency = 2
- W(SO2) sample: Determined weight of SO2 in sample [mg SO2]
- Vsample: Titrant volume for sample [mL]
- c(SO2)sample: Determined SO2 concentration in sample [ppm SO2]
- msample: Sample volume [mL]
The modified Monnier-Williams method is based on pH titration and involves distilling the sample with an acid mixture which converts sulfite ions to steam-volatile sulfur dioxide
Eggcule points out that this method is great for many applications in the food and feed industry, but that there is a more effective method for samples like wine or juice that contain volatile acids like acetic acid. Instead, Eggcule suggests that the client use an iodometric titration method based on back titration with a redox electrode that was patented by BUCHI. Eggcule explains that the volatile acids can interfere with the Monnier-Williams method that involves heating the sample, which can cause the loss of volatile acids and SO2. In contrast, the iodometric titration is performed at lower temperatures and minimizes the loss of volatile components. The iodometric titration method is quicker and offers greater sensitivity and selectivity in such cases and the redox electrode provides a clear endpoint that is more effective than other methods. Eggcule then provides the steps for the BUCHI method that he believes will be just what the client needs.
- Prepare the BUCHI SO2 absorption glass set (11073599) by filling it with 50 mL of deionized water in the first receiving vessel and 30 mL of ethanol into the second receiving vessel, pipette 0.05 M iodine solution into the first receiving vessel
- Attach the SO2 absorption glass set to the distillation unit and start steam distillation with an acid mixture dosed into the sample tube
- The steam distilled SO2 reacts with the iodine solution: SO2 + I2 + 4 H2O → 2 HI + SO42- + 2 H3O+
- After distillation, pour solutions from both receiving vessels into a 700 mL beaker, rinse the receiving vessels + connection piece thoroughly, and collect everything in the beaker
- Add 2.0 mL of 0.5 M sulfuric acid to the beaker for acidification and fill up to 600 mL total volume with deionized water
- Start titration with sodium thiosulfate solution and redox electrode to titrate back the excess of iodine with sodium thiosulfate solution: I2 + 2 Na2S2O3 →2 NaI + Na2S4O6
Eggcule highlights the importance of properly rinsing the glass absorption set when transferring the sample solution to the beaker for titration and that the acid pump needs to be flushed afterward to make sure the acid mixture will not stay overnight in the pump. He then listed the following calculations used to determine the mass and concentration in the sample:
Mass of SO2:
Concentration of SO2:
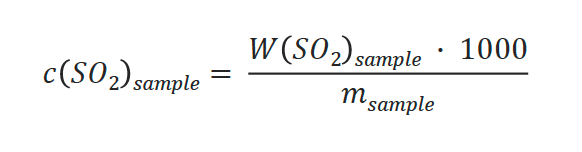
- W(SO2): Weight of SO2 calculated from Vsample [mg SO2]
- M(SO2): Molar mass SO2 = 64.0648 [g/mol]
- Vblank: 0.01 mol/L thiosulfate consumption for blank [mL]
- c: Concentration of thiosulfate (titrant) [mol/L]
- z: Redox valency of thiosulfate = 2
- W(SO2) sample: Determined weight of SO2 in sample [mg SO2]
- Vsample: Titrant volume for sample [mL]
- c(SO2)sample: Determined SO2 concentration in sample [ppm SO2]
- msample: Sample volume [mL]
The BUCHI method involves a patented iodometric titration method based on back titration with a redox electrode which is quicker and offers greater sensitivity and selectivity
Holmes is delighted with the work of his detectives but thinks that Vinnie might struggle with these techniques, so he sends Eggcule to the vineyard to assist with the determination. “I also have an extremely great way of determining whether the right amount of sulfites have been used in wine, so that the taste hasn’t been negatively affected” says Eggcule. The detectives are all keen to hear about the method. Eggcule says “Why you drink it, of course!” And with that, Eggcule went skipping out the door feeling like the luckiest detective in the world.