Freeze drying
Overview of the freeze-drying process
Freeze drying is the gentlest process to dry various types of perishable materials. The principle of freeze drying is based on the direct transition of a substance from the solid to the gaseous state, called sublimation. Initially, the product is frozen and then dried by sublimation in an environment of reduced pressure, without being allowed to thaw.
Benefits of the lyophilization process
Product stability is massively increased by reducing its water content due to the direct link between water presence and biological and chemical activity which are mainly responsible for product degradation. Compared to other dehydration methods, freeze drying causes less product damage and avoids shrinkage or agglomeration of the material. Because of this, the freeze-drying method is ideally suited to:
- Preservation of delicate material against degradation or decomposition
- Preservation of product characteristics and initial shape
- Conservation of products that require fast rehydration or conditioning of product for further use
The initial freezing process creates ice crystals within and on the surface of the product. By turning into ice, the individual water molecules lock up into a well-defined grid. As the water molecules sublimate from the product, they leave little pores and gaps within the product and thus maintain its shape and structure. Rehydration of the product is therefore quick and simple, a particularly important feature in pharmaceutical applications. Freeze dried products can last many years at room temperature if they are well sealed and protected against moisture and oxygen.
Vaccines, dried fruits and vegetables, dried mushrooms or soluble coffee are common freeze-dried products available in everyday life.
Principle of the lyophilization process: Thermodynamic basics
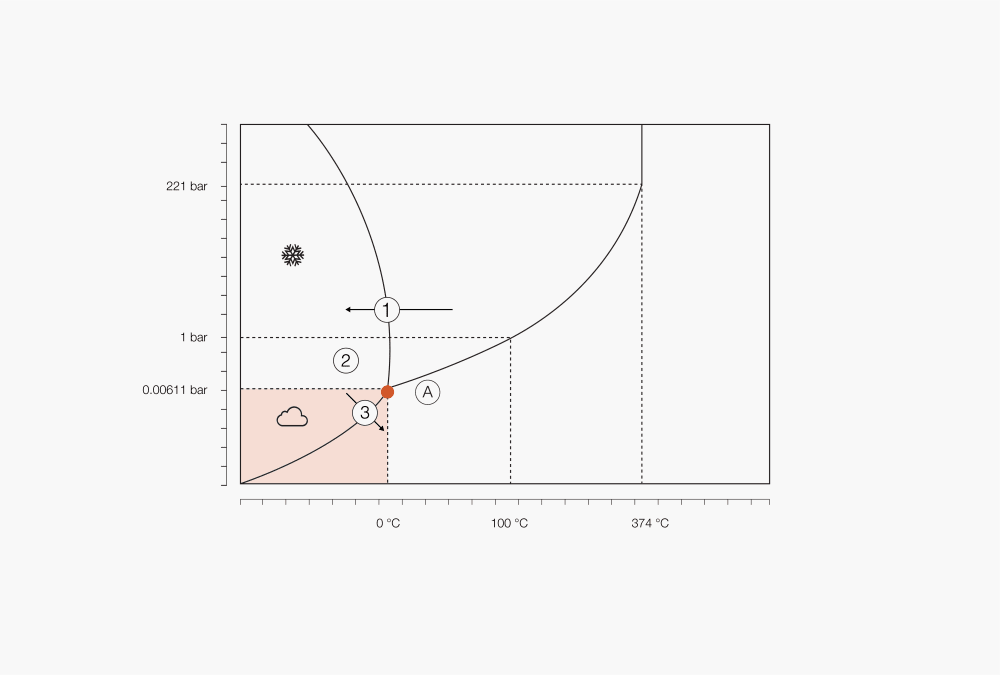
Figure 1 : Phase diagram of water
① Choose freezing temperature depending on solvents and solute
② Start of sublimation by lowering the pressure
③ Below triple point freeze drying starts
Ⓐ Triple point
Depending on pressure and temperature, any substance may be present in three phases – solid, liquid and gaseous. The relationship between pressure and temperature for a defined substance is shown in so-called phase diagrams. When a solid is heated under constant pressure above the triple point, it will reach the melting point and liquefy. Further heating will lead to an increase in temperature until the boiling point is reached, the liquid will then start boiling, changing into a gas.
When a similar process occurs with temperature and pressure below the triple point (for water, 6.11 mbar), the material will not melt but sublimate instead. The heat energy supplied to the sample at low-pressure transfers enough energy for thawing, however the pressure is too low for liquid formation and the solvent will therefore sublimate into gas.
Since the phase of a substance is determined by both the heat and the pressure, the temperature at which boiling or vaporization occurs is set by the pressure. Reducing the pressure by applying a vacuum can therefore lead to a decrease in the solvent boiling point and to a vaporization occurring at lower temperatures. Low-pressure systems are commonly used for heat-sensitive samples to decrease the boiling point so that vaporization occurs at a lower, safer temperature. A similar approach can be undertaken for sublimation processes.
Effects of pressure and temperature on the freeze-drying method

Figure 2: Freeze drying steps
■ Pressure
■ Product
■ Shelf
■ Condenser
Ⓐ Lowering product and shelf temperature for an optimum process
Ⓑ Lowering the pressure and rising the shelf temperature facilitate sublimation as well as desorption in secondary drying
Ⓒ Ice condenser temperature determines the actual condenser capacity to collect vapors
The crucial parameters governing your freeze-drying equipment are pressure and temperature. A typical freeze-drying process involves two stages – freezing and primary drying. For some samples a secondary drying might be required in order to remove solvent molecules tightly attached to the sample and reduce moisture further. Each process step has distinctive requirements in terms of pressure and temperature depending on sample characteristics.
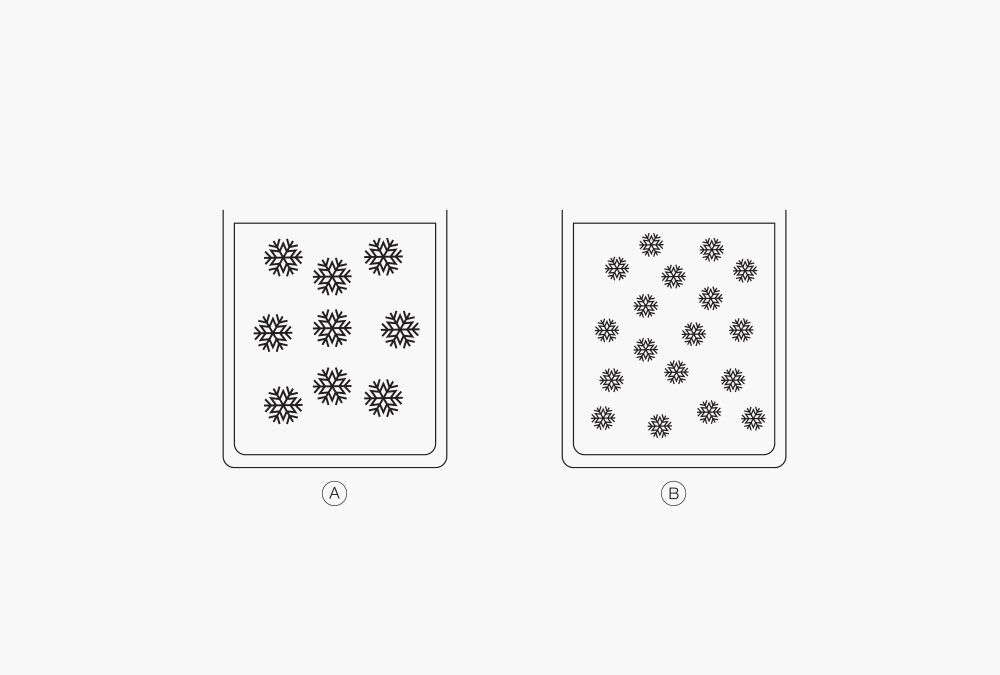
Figure 3: Differente ice crystal size depending on freezing speed.
Ⓐ Slow freezing
Ⓑ Fast freezing
Most liquid products, or formulations, freeze by forming ice crystals. Size and shape of the ice crystals depend on the cooling speed and define the freeze drying ability; rapid cooling (liquid nitrogen) results in small ice crystals while slower cooling (deep freezer) leads to larger ice crystals. In terms of freeze drying, small ice crystals are more challenging to remove from the product than large ones. Yet, the freezing temperature of a formulation is defined by its characteristics and composition.
Eutectic and amorphous mixtures in the lyophilization process
Formulations can generally freeze in two different ways for eutectic and amorphous mixtures.
Eutectic mixtures
Eutectic mixtures contain substances that freeze at lower temperatures than the water surrounding them. When cooling a eutectic mixture, water is the first to separate from the substances and it freezes to ice. The formulation may then appear frozen but the remaining substances are actually still liquid. They form concentrated areas that freeze eventually at temperatures below the freezing point of water.
The temperature where all components of the mixture are properly frozen is called eutectic temperature. This is the critical temperature of the formulation and the maximum temperature the formulation can endure during the freeze-drying process. Applying vacuum to an incompletely frozen eutectic mixture, may result in the destruction of the product as unfrozen components expand when placed under vacuum.
Amorphous mixtures
The other class of mixtures is amorphous and form glassy states when frozen. With decreasing temperature, the formulation becomes more and more viscous and eventually freezes to a vitreous solid at the glass transition point. For amorphous products, the critical point in terms of stability is called collapse temperature. The collapse temperature is typically slightly lower than the glass transition point. Amorphous products are generally very challenging to freeze dry with freeze-drying equipment.
Primary drying during the lyophilization method
The first drying phase – the primary drying - removes the bulk of water within the product by sublimation. The temperature of the product is defined by the pressure in the drying chamber and the heat input must be carefully controlled. The ideal product temperature is as high as possible to maximize the vapor pressure difference between the sample and the condenser, though at the same time it must remain below the product’s critical temperature to preserve the frozen character. Above this temperature the product structure collapses leading to shrinkages or cracks.
Ideally, the lyophilization process is performed at temperatures just below the critical temperature. The primary step of the freeze-drying process occurs as follows:
- The drying chamber pressure is decreased to activate the drying process
- The prevailing pressure and temperature readings are now both below the triple point
- By using heated shelves, the set temperature is slowly approached at a defined heating rate
- Sublimation creates water vapor in the drying chamber
- If not removed from the system, the water vapor equilibrates and no further ice particles sublimate
- The vapor particles are removed by means of the ice condenser, a cooling device running at temperatures far below the critical product temperature.
The rate of sublimation is basically defined by the difference in vapor pressures: the vapor pressure over the product on one hand, and the vapor pressure over the ice condenser on the other hand. Generally, the larger the difference, the faster the sublimation; the closer the product temperature is to the triple point, the larger is the pressure difference.
The vast majority of the water should be removed by the end of the primary drying phase by the freeze-drying equipment. The residual moisture content of the product may now be 5 – 10% due to water bound to the matrix. At this stage, ice should not be present anymore.
Secondary drying during the freeze-drying process
The secondary drying step removes the adsorbed water molecules by desorption. In order to achieve ideal conditions for desorption, the lowest possible pressure as well as a further increase of the shelf temperature is required. Again, product stability must be considered when choosing the shelf temperature. Secondary drying is usually performed for shorter time periods. At the end of secondary drying, the product moisture content should be in the range of 1 – 5%.
The lyophilization process in the pharmaceutical industry
The freeze-drying process is usually the favorite choice for the preservation of a wide range of pharmaceuticals, mainly when stability in the liquid state is not adequate, storage requirements are too rigorous or when the product is required in solid form. It is well suited for formulations that do not require further processing after drying since they can be filled directly in vials, which can be sealed in the drying after the cycle, in order to avoid potential contaminations.
| Benefits of freeze drying | Limitations of freeze drying |
|---|---|
Low process temperatures | Requires a large initial investment for freeze drying equipment |
High product yields | Long processing times |
Great product uniformity | Limited upscaling possibilities |
High quality in terms of activity, water content and stability |
|
Accurate control of the process enables the production of a product of the highest quality since it minimizes risk of intrinsic products properties such as collapse, eutectic melt or glass transition temperatures being exceeded.