Kjeldahl Nitrogen Determination
Nitrogen / Protein Determination Procedure according to Kjeldahl
The Kjeldahl process is an established method which is widely used for all kinds of food samples but also for environmental, chemical and pharmaceutical samples. It involves a digestion step to decompose proteins and other nitrogen-containing species and is followed by steam distillation to isolate the ammonia for nitrogen quantification.

The Kjeldahl Process
Proteins are some of the most important nutritional components and are present in almost all food and animal feed products, where they serve as a reliable quality feature. For food products, the protein content must be declared on the product nutritional information for consumers. This declaration is statutory in national and international laws for food producers. By determining the Total Kjeldahl Nitrogen (TKN), the protein content is calculated directly from the nitrogen present in the sample. TKN analysis also determines the content of both organic and inorganic forms of nitrogen in the respective samples and is applicable to a broad variety of Applications. In food analysis, total volatile basic nitrogen (TVBN) is used to determine the freshness of fish and seafood products.
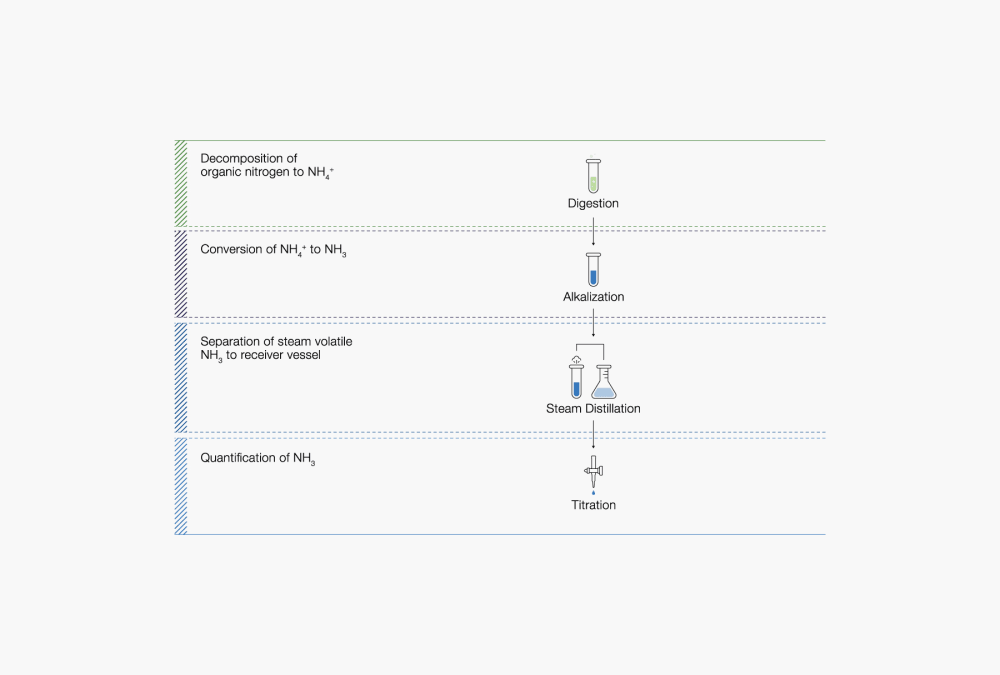
Fig.1 The three major steps in Kjeldahl nitrogen determinations include digestion, steam distillation and titration.
Step 1: Digestion
The analysis starts with the acid digestion of the sample by using a Digester, converting organic nitrogen to ammonia. The sample needs to be boiled in concentrated sulfuric acid and Kjeldahl Tablets containing potassium sulfate and copper catalyst to convert the organic nitrogen to ammonia (Fig. 2). The Digester is coupled to a Scrubber to remove corrosive fumes to achieve highest safety in the lab.
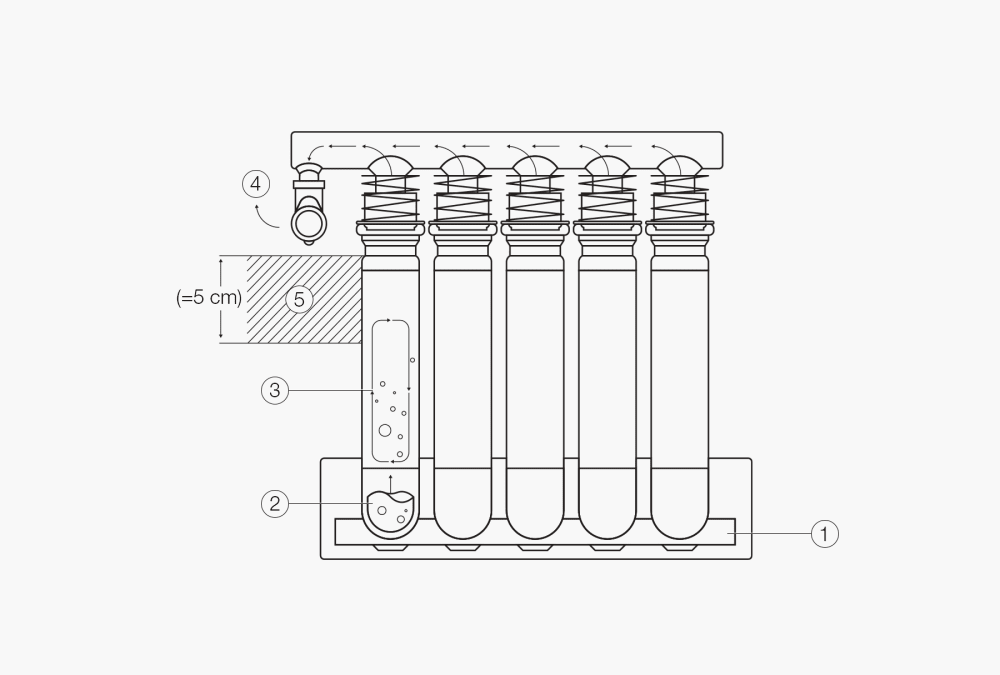
Fig. 2 The digestion process via block heating.
The aluminum block ① generates high temperatures in the samples ②
The sample is digested in constantly boiling sulfuric acid
Hot acid fumes rise into the condensation zone ③ , and condense and rinse back down to the sample creating a constant reflux
Residual fumes ④ which escape the condensation zone ⑤ are highly corrosive and must be withdrawn and efficiently neutralized (e.g. with the Scrubber K-415)
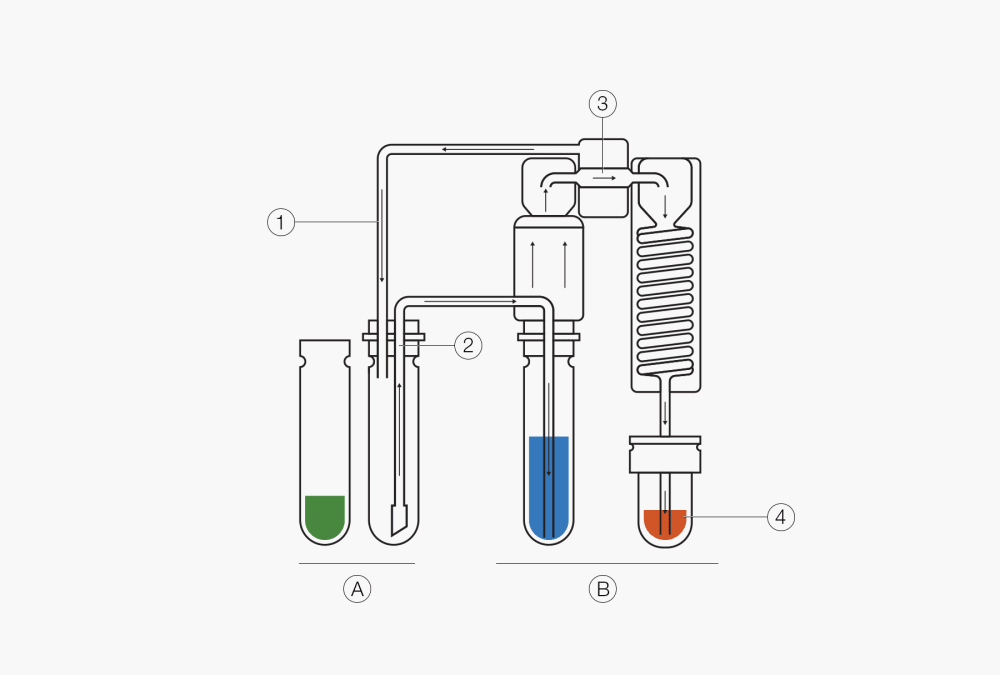
Step 2: Steam Distillation Procedure and Titration
The second part of the method involves steam distillation with a suitable Distillation Unit. The pH of the digestate must be raised to 9.5 by adding concentrated sodium hydroxide in this alkalization step. At this pH, ammonia gas forms. With the Reaction Detection Sensor, the amount of sodium hydroxide is automatically optimized which saves resources and costs. The ammonia gas is then transferred through steam distillation into the acidic absorbing solution, namely diluted Boric Acid, and converted to ammonium (Fig. 3). Nitrogen concentrations within the receiving solution can then be determined using classical potentiometric or colorimetric electrode determination methods.