Supercritical Fluid Chromatography
The Green Standard for Fast Compound Isolation
Supercritical Fluid Chromatography has been researched and applied for several decades. The technique has dramatically advanced over the last decade. This trend towards SFC came about because it opened the door to a much better world for any chemistry lab.
What is SFC?
A novel separation technique.
SFC (Supercritical Fluid Chromatography) is a separation technique similar to HPLC (high-pressure liquid chromatography) but uses supercritical fluids as a mobile phase. Therefore, to operate SFC, it is necessary to maintain temperature and pressure above the critical level of the mobile phase throughout the column.
The difference in the setup of an HPLC and SFC instrument is shown below.
Prep HPLC
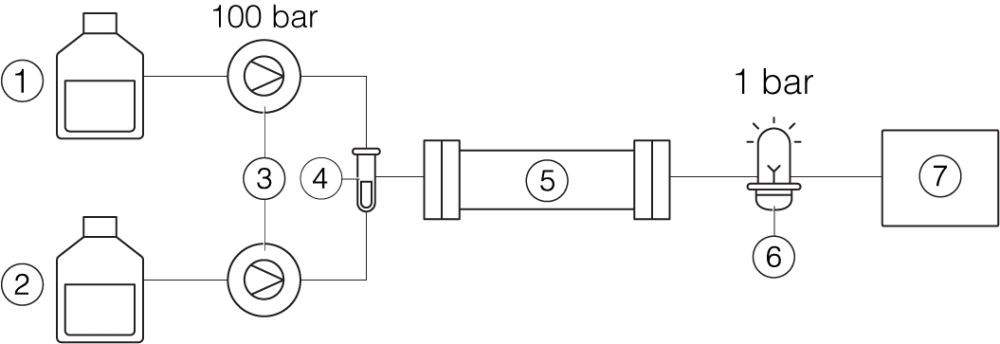
① Solvent A ② Solvent B ③ Pumps ④ Sample ⑤ Column ⑥ UV detector ⑦ Fraction collector
Prep SFC
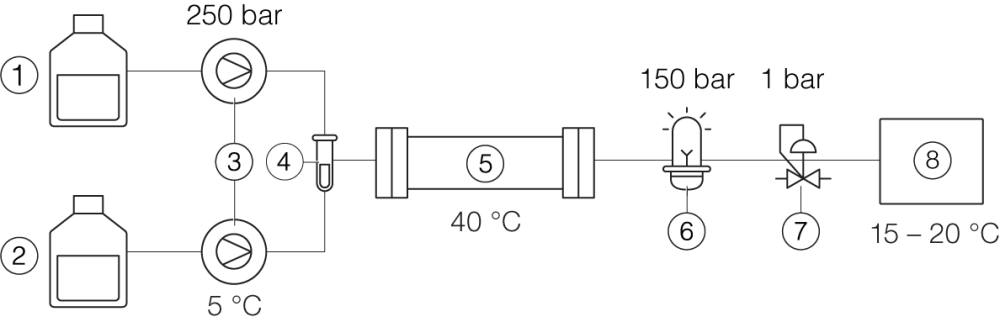
① Solvent A ② Liquid CO2 ③ Pumps, incl. chiller ④ Sample ⑤ Column, column oven ⑥ UV detector ⑦ Back pressure regulator ⑧ Fraction collector
The role of CO2
Carbon dioxide is the most commonly used supercritical fluid for several reasons. It has a low critical temperature and pressure (31 °C and 73.8 bar), is highly inert under most conditions, non-flammable and it has minimal reactivity, and high purity at a low cost. Carbon dioxide is also miscible with many high polar organic solvents, in contrast to n-hexane, which offers low polarity compared to supercritical carbon dioxide. These factors allow for SFC to achieve a wide variety of separation patterns.
What is a supercritical fluid?
Depending on temperature and pressure conditions, substances can be in a solid, liquid, or gaseous state. If a liquid or gas is used above its critical temperature and pressure, it changes to a supercritical fluid. The characteristics of supercritical fluids are intermediate between those of gases and liquids. A supercritical fluid can be considered a dense gas.