Spray drying
What is spray drying?
Since the 1940s, spray drying has been a robust and widely used manufacturing process and applications are found in all major industries ranging from industrial chemistry, pharmaceutical, biotechnology to food industry. Dry milk powder, instant soups, solid dosage form pharmaceuticals, instant coffee, detergents and dyes are just a few examples of spray dried products on the market.
Spray drying is an elegant method to dry solid substances from aqueous or organic solutions, emulsions and suspensions. During the process, a spray dryer atomizes a liquid feed into fine droplets and evaporates the organic solvent or water by means of a hot drying gas.
Benefits of spray drying
Spray drying can be considered a high throughput process since it is drying very quickly compared to other drying techniques. It provides the advantage of weight and volume reduction. The transformation of a liquid product into a dry powder is done in a single step, which makes the method advantageous in terms of costs, scale-up and process simplification. Its gentle process can handle a broad spectrum of compounds including heat sensitive substances such as biologic products, pharmaceuticals or food nutrients. The product properties and quality can be effectively controlled; spherical and relatively uniform particles can be readily produced. The powder can be fully engineered and processed into tablets/capsules without milling or other secondary processing, moreover, most temperature sensitive substances like enzymes, proteins, antibiotics, etc. can be spray dried without major loss of activity. Spray drying also works under inert atmospheres, needed to protect the products, or for organic based liquids without process risks.
When compared to other drying techniques, such as freeze-drying, the spray drying process is shorter and cheaper since it does not involve deep freezing of the samples and requires less energy consumption. Some researchers have explored the use of spray-drying as an alternative method to freeze-drying.
Limitations and benefits of laboratory scale spray drying
Despite the many advantages, some challenges arise when applying this technology. Due to a loss of product on the wall of the drying chamber and into the exhaust air, yields in laboratory scale experiments are not always optimal and are reported to be in the range of 20-70 %. At industrial scale however, yields increase with larger scale setups since the lost fraction is a smaller part of the production volume. Therefore the limitation in yields will mainly only occur in the lab during the development phase and will improve after in product scale. Due to the fact that two-fluid, three-fluid and ultrasonic nozzles have to be used and the limitations of the cyclone technology, makes the production and the recovery of sub-micron particles difficult. This phenomenon has to be considered in the development of drug delivery systems such as intravenous administrated pharmaceuticals. Laboratory scale spray drying is also limited to produce particles with a size range above 50 μm – similar to those produced at large scale. This needs to be taken into account during lab scale screening since it could lead to some issue later during scale up when dissolution profile of particles and powders are important parameters. On the positive side laboratory scale spray drying allows to handle small samples in short time. Cleaning time during runs are much shorter than with industrial or pilot scale size instrument. This allows to run many more experiments in the same time and therefore optimize the formuation and the used parameters. Also the availability of the sample might be limited and using less for an experiment is a clear advantage. The fact the laboratory scale spray dryers are made of glass allows to observe the drying process of the sample and optimize the process when needed.
Spray drying in different industries
Over the past years, spray drying has gained importance as a method to produce dry powder, due to its continuous, gentle, single step, scalable process.
It is successfully used in food-, chemical- and pharmaceutical-industries (Table 1) for production and research.
Table 1 : Applications of spray drying
| Food applications | Chemical | Pharmaceutical |
|---|---|---|
Milk powder, eggs, coffee | Ceramic materials, nano materials, batteries and material sciences | Pulmonary delivery, |
Infant food | Detergents, soaps,... | Biopharmaceutical products |
Animal feed | Pesticides, herbicides, fungicides, insecticides, fertilizers, | Antibiotics, vaccines, vitamins, yeast, |
Encapsulation of flavors | Pigments, paints and dyes |
|
Bioactive compounds, neutraceuticals | Cosmetics |
|
Spray drying process in the food industry
In food technology, products such as coffee, dried eggs, powdered milk, animal feeds, cake mixes, infant formulas, starch derivatives, nutritional oils or yeasts are usually manufactured by spray drying. Spray drying yields products with good solubility properties, keeps flavor loss to a minimum, allows processing of heat sensitive foods with high retention of their nutritive content, and has an economic potential for scale up.
Spray drying process in the chemical industry
In the chemical industry, products such as cosmetics, detergents, pesticides, herbicides, pigments and dyes or ceramic materials are commonly obtained by spray-drying. Reducing the size of particles found in dyes enables a more consistent and convenient dispersion into paint. Additionally, granulation via spray drying can improve flow and distribution of molecules and particles in the final product. In material science, spray drying is mainly applied to granulate nanoparticles into sub-micrometer- to micrometer size particles in order to obtain free-flowing powders. These powders are then further processed to batteries, bioceramics, or used for advanced materials research purposes.
Spray dryed nanomaterials (nanoparticles, nanosuspensions) are often used as:
- Coatings in turbine engines, automotive parts, photocatalytic and biological implants (titania, alumina, zirconia, yttria coatings)
- Advanced Ceramics of metal carbides, nitrides or borides (eg. new super conducting ceramics)
- Toners and Magnetic Tapes (eg. ferrites)
Spray drying process in the pharmaceutical industry
In pharmaceutical industries, applications include the spray drying of excipients, of pure drugs or the encapsulation of drugs. Spray drying is widely used to manufacture products with defined physical and chemical properties for controlled drug release, or to improve the dissolution of low water soluble drugs such as carbamazepine, ibuprofen or ketoprofen.
Applications of the spray drying process
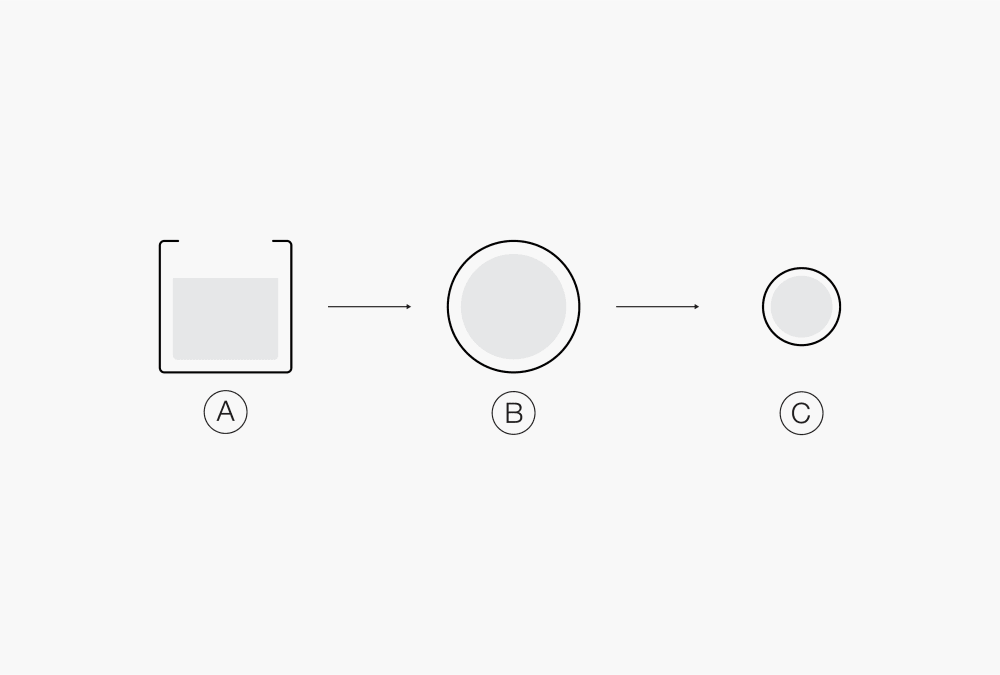
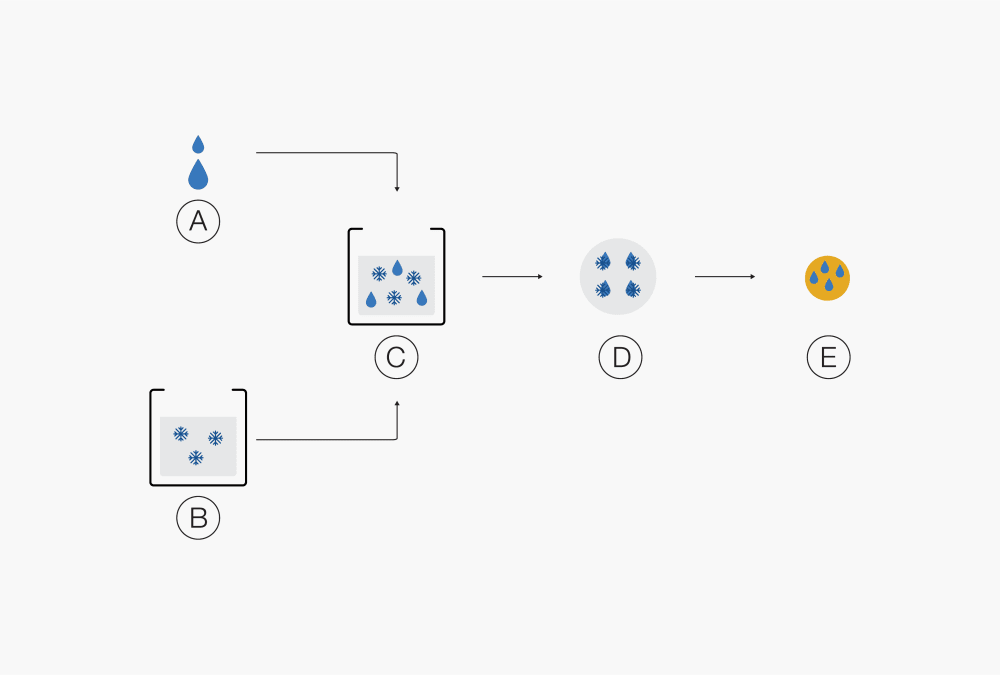
Ⓐ Liquid product
Ⓑ Droplets
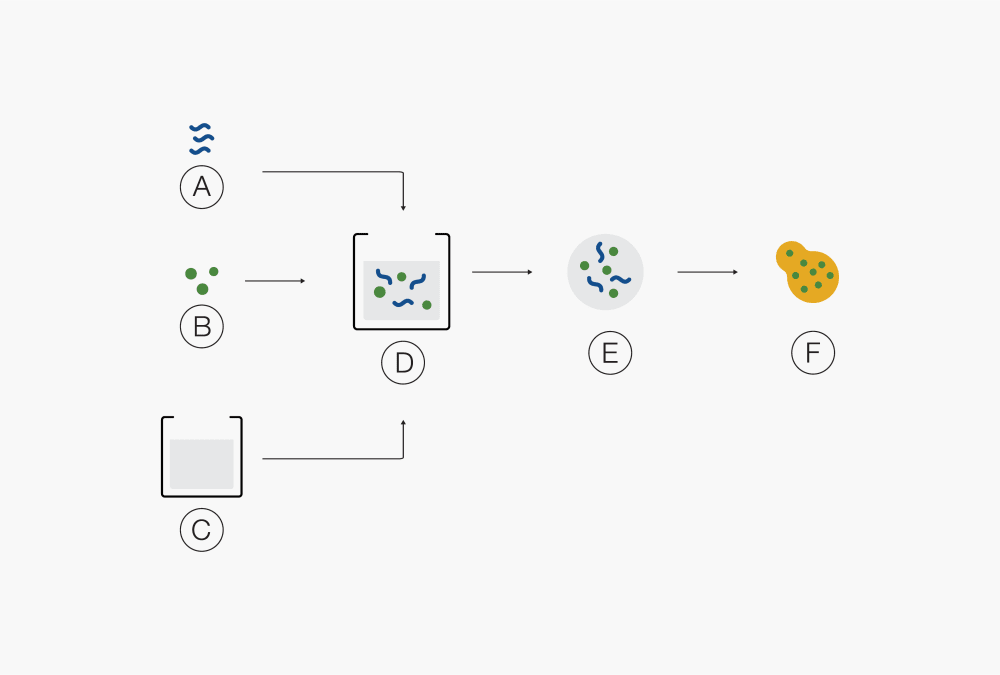
Ⓒ Solid particlesⒶ Polymer Ⓓ Solution of drug and polymer in solvent B
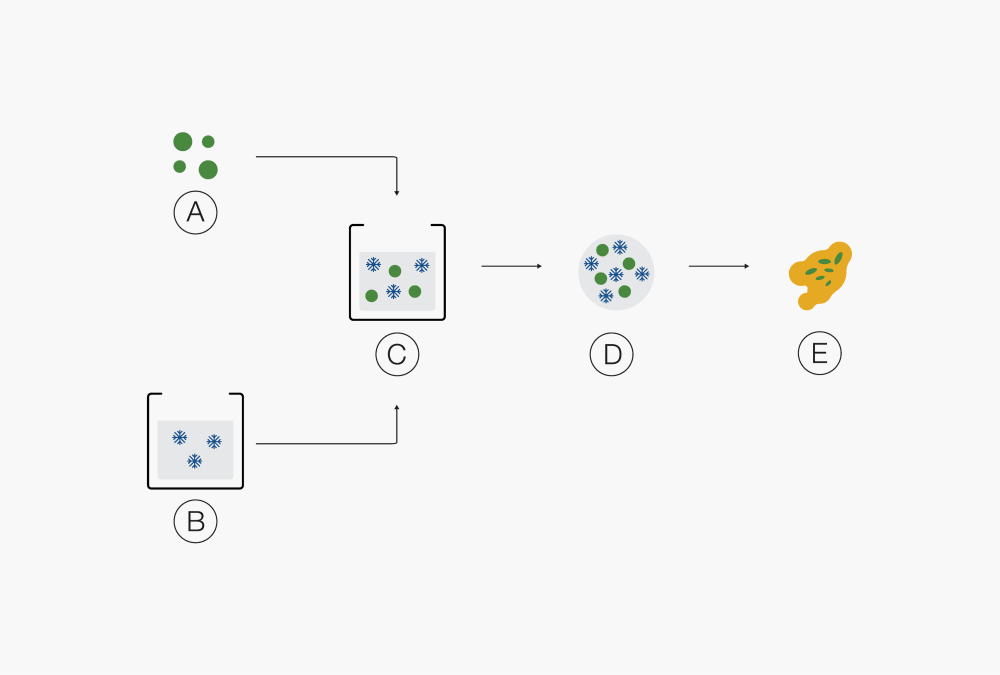
Ⓑ Drug Ⓔ Droplets
Ⓒ Solvent Ⓕ Molecular mixture of API and polymer(s)Ⓐ Solid product Ⓓ Droplets
Ⓑ Solvent Ⓔ Solid particles
Ⓒ Solution of the solid product
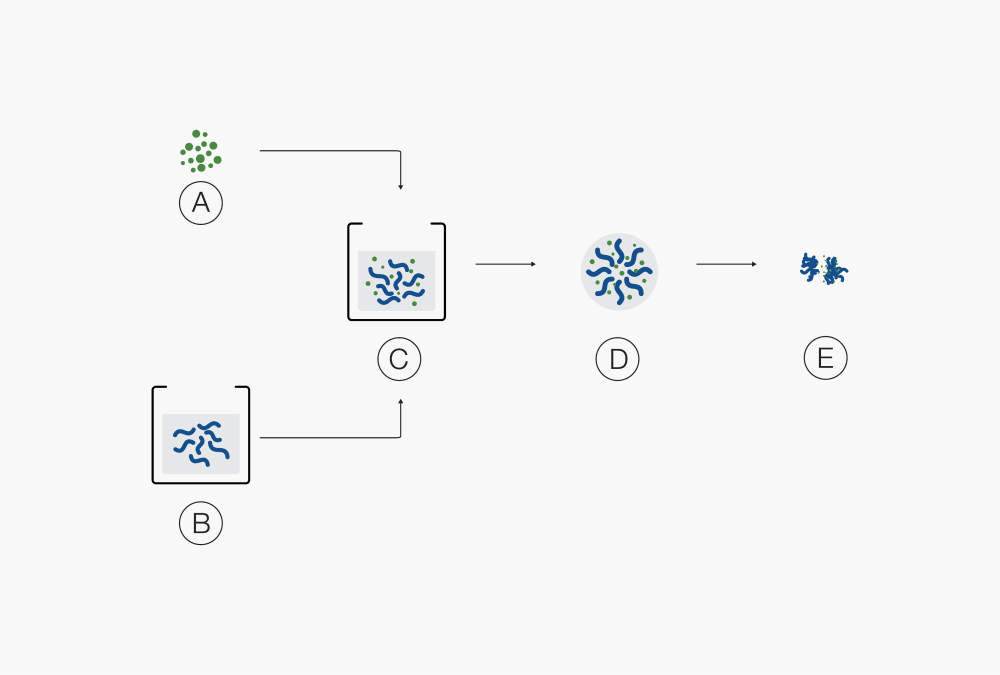
dissolved in the solventⒶ Solid products Ⓓ Droplet
Ⓑ Binder dissolved in solvent Ⓔ Agglomerate of solid particles
Ⓒ Suspension of solid particles
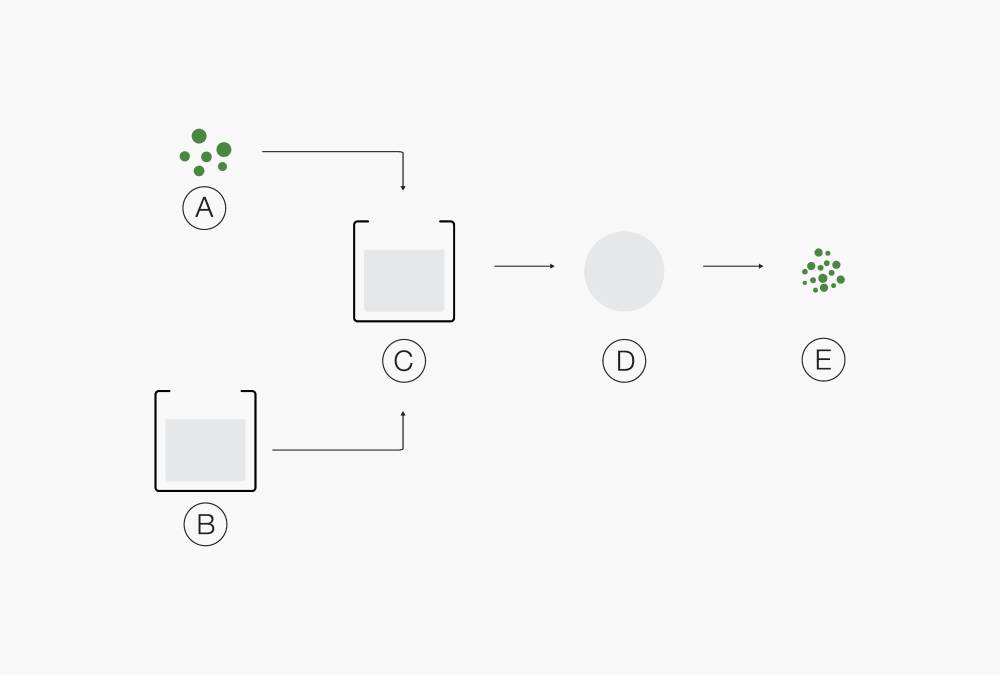
in binder solutionⒶ Liquid product Ⓒ Emulsion
Ⓑ Solution of carrier Ⓓ Droplets
and filmogen Ⓔ Solid particlesⒶ Solid products Ⓓ Droplet
Ⓑ Solution of carrier and filmogen Ⓔ Solid particles
Ⓒ Dispersion
Although many techniques were developed, spray drying is one of the most common technologies to obtain granulated substances due to its single step process, its mild process conditions, and its scalability In general, the application for spray drying can be divided into distinct areas as shown above. Amongst them drying, structural change, encapsulation or amorphous solid dispersion.
How does spray drying work
Spray drying is accomplished by dissolving, emulsifying or dispersing the core substance in a solvent or in a solution of carrier material. The material is then atomized and sprayed into the drying chamber where a hot stream of drying gas will help evaporate the solvent to produce dry solid particles that will further be separated from the gas stream and collected using centrifugal forces with a cyclone.
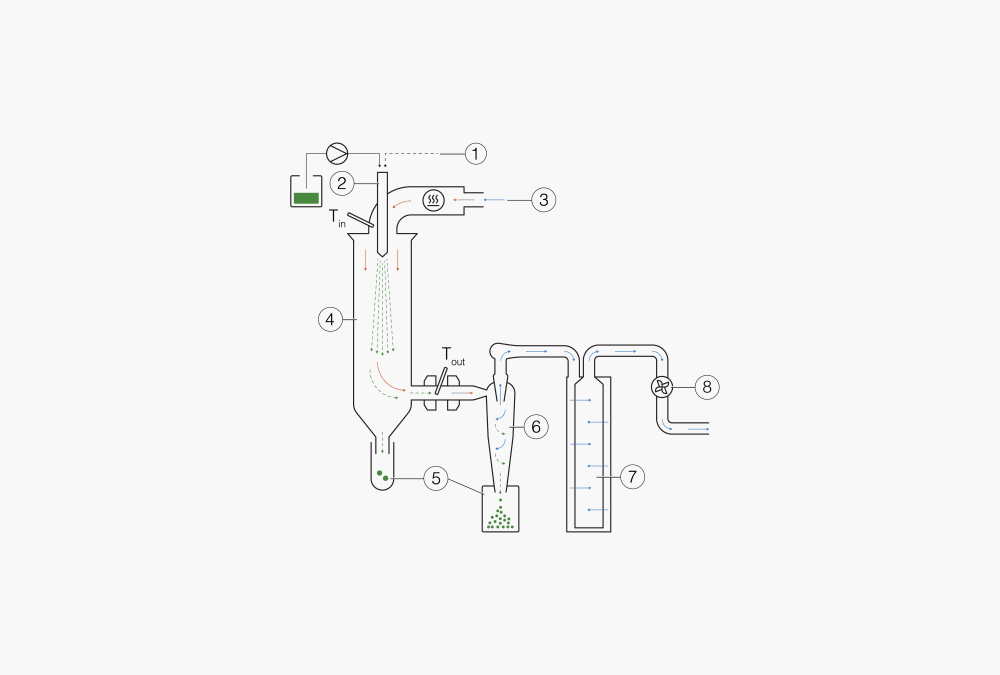
Figure 2: Functional principle of conventional spray dryer
① + ② Droplet formation: Two-fluid nozzle for the S-300
③ Heating: Heat the inlet air to the desired temperature (max. 250 °C)
④ Drying chamber: Conductive heat exchange between drying gas and sample droplets.
⑤ Particle collection in two possible places
⑥ Particle collection: Cyclone technology
⑦ Outlet filter: Collection of finest particles to protect the user and the environment.
⑧ Drying gas: Delivered by aspirator
Particle shapes and structures
As illustrated in Figure 3, several types of particles can be produced from a spray drying process. Their morphology include dense, hollow, porous composite or encapsulated structures with spherical, wrinkled, shriveled collapsed or cenospheres (doughnut-like) shapes.
As a general rule, slow drying leads to more compact particles, whereas fast drying yields the formation of hollow particles.
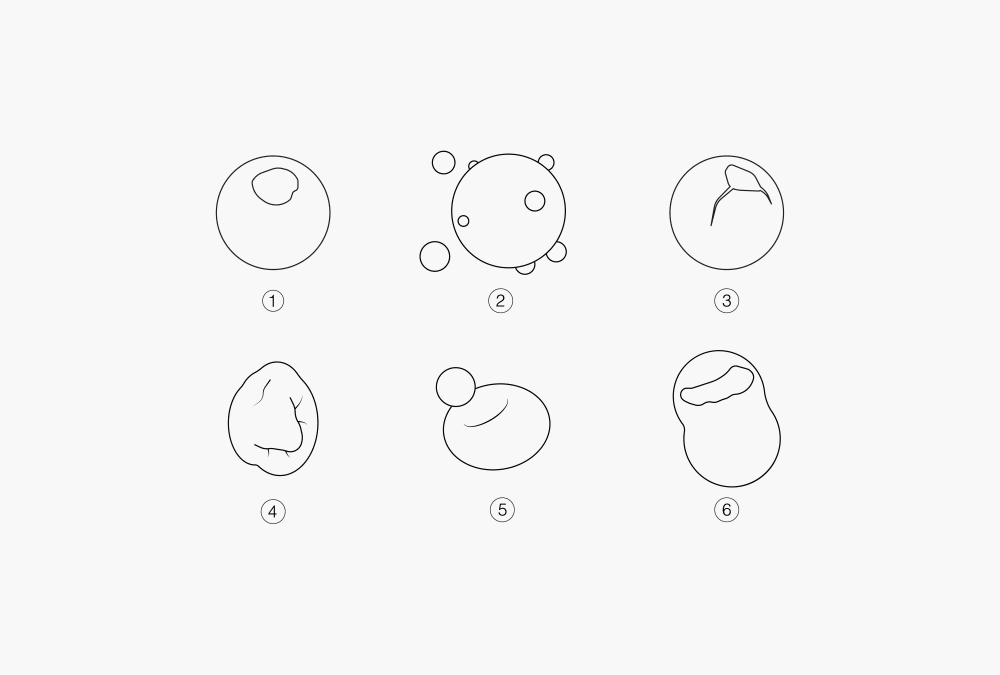
Figure 3: Particle shapes and structures produced with spray drying
① Solid particles ② Satellites ③ Hollow particle ④ Shriveled particle
⑤ Cenosphere ⑥ Disintegrated particle
Optimizing the spray drying process
The results of the spray drying method highly depend on material properties, equipment design and correlation of the process parameters. Those factors have an influence on the quality of the final product in terms of morphology, residual moisture and particle size. Optimization of the process is usually achieved by a “trial and error” approach, however, understanding the basic guidelines of spray drying can help the user with efficient use of the equipment.
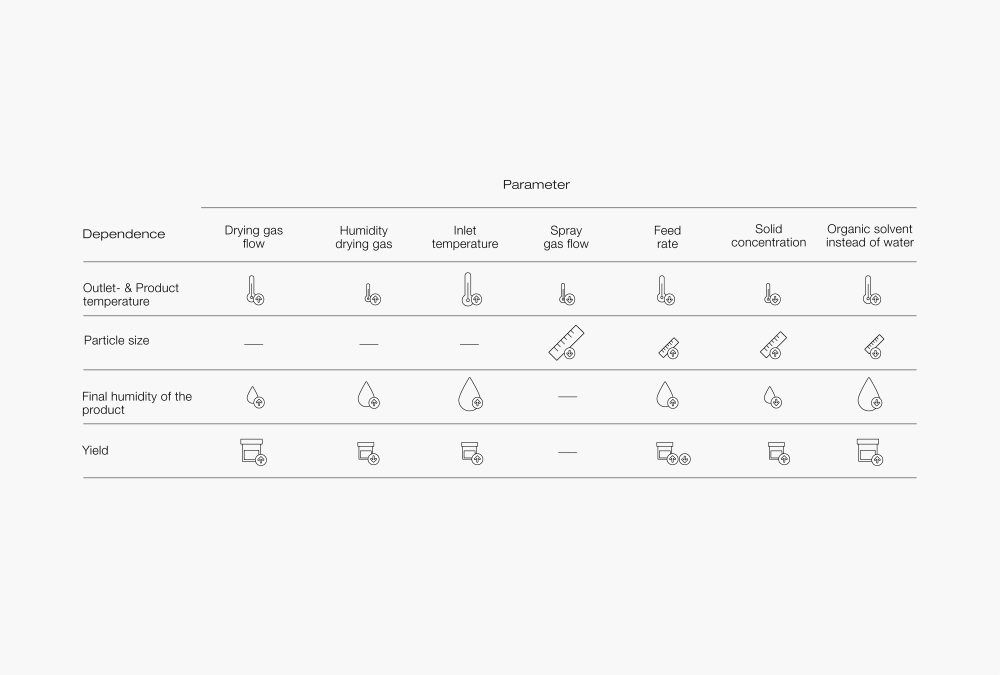
Figure 4 : This table shows the dependency of output parameters vertical axis when one of the input parameters, horizontal axis, is increased. The size of the picture shows the impact of the change and the arrow shows the direction.
General guidelines for optimizing spray driyng, microencapsulation:
The peristaltic pump feeds the spray solution to the nozzle. The pump rate affects the difference between the inlet and the outlet temperature and the final particle size:
- A higher gas spray flow rate leads to smaller droplets and accordingly to smaller dried particles.
- An increase in solid concentration in the feed results in larger and more porous dried particles. The solid concentration depends strongly on the application.
An increase in the feed flow rate, at constant atomizing gas flow rate, results to an increase in droplet size
The higher the throughput, the more energy is needed to evaporate the droplets into solid particles. Thus, the outlet temperature decreases. When the pump rate is too high, wet and sticky particles, which adhere to the spray chamber wall are obtained. An increase of the feed pump rate lowers the outlet temperature and increases the temperature difference between the inlet and the outlet.
Reducing the pump rate, while keeping inlet temperature and aspirator rate constant, results in a drier final product
- The inlet temperature is the temperature of the heated drying gas. A higher inlet temperature is favorable in terms of achieving higher throughput; however, a lower value prevents degradation or losses of active compounds.
- The outlet temperature is determined by the heat and mass balance in the drying cylinder and cannot be regulated. It is influenced by the following parameters: Inlet temperature, Aspirator flow rate/speed, Feed flow rate, Concentration of the material being sprayed
- A higher aspirator rate leads to a higher degree of separation in the cyclone. A lower aspirator rate results in a lower residual moisture content.
- The residence time is important with respect to the complete drying of the droplets and to control of the particle temperature so that aroma loss or thermal degradation of heat sensitive materials can be minimized. The typical residence time for a laboratory scale spray dryer is of 0.2-0.35s
- The glass transition temperature Tg is the temperature above which the structure of the matrix switches from a rigid glassy state to a rubbery state. This is associated with product stickiness. The Tg of the feed depends on the constituent solutes in the feed. For example, water is known to depress Tg considerably, while high molecular weight components such as maltodextrin can be used to make Tg of the feed higher. In order to avoid product stickiness and associated problems, such as caking and lumping of the product during packaging, the outlet temperature should not exeed Tg during the process.